Estimated Health Outcomes and Costs of COVID-19 Prophylaxis With Monoclonal Antibodies Among Unvaccinated Household Contacts in the US
- PMID: 35452104
- PMCID: PMC9034404
- DOI: 10.1001/jamanetworkopen.2022.8632
Estimated Health Outcomes and Costs of COVID-19 Prophylaxis With Monoclonal Antibodies Among Unvaccinated Household Contacts in the US
Abstract
Importance: The COVID-19 pandemic has led to more than 900 000 deaths in the US and continues to disrupt lives even as effective vaccines are available.
Objective: To estimate the health outcomes and net cost of implementing postexposure prophylaxis (PEP) with monoclonal antibodies (mAbs) against household exposure to COVID-19.
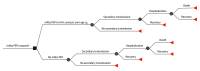
Design, setting, and participants: This study is a decision analytical model of results from a randomized clinical trial of casirivimab with imdevimab administered as subcutaneous injections to unvaccinated, SARS-CoV-2-negative household contacts of people with confirmed COVID-19 with complementary data on household demographic structure, vaccine coverage, and confirmed COVID-19 case counts. The study used US data from May 2021 for a simulated population of US individuals of all ages within low-transmission or high-transmission scenarios.
Exposures: Age, sex, race, ethnicity, and COVID-19 vaccination status.
Main outcome or measures: Symptomatic infection, hospitalization, death, and net payer cost of monoclonal antibody PEP for COVID-19.
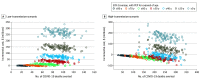
Results: In a month of transmission intensity similar to that of May 2021, a mAb PEP program reaching 50% of exposed, unvaccinated household members aged 50 years and older was estimated to avert 1820 symptomatic infections (95% uncertainty interval [UI], 1220-2454 symptomatic infections), 528 hospitalizations (95% UI, 354-724 hospitalizations), and 84 deaths (95% UI, 55-116 deaths) in a low-transmission scenario and 4834 symptomatic infections (95% UI, 3375-6257 symptomatic infections), 1404 hospitalizations (95% UI, 974-1827 hospitalizations), and 223 deaths (95% UI, 152-299 deaths) in a high-transmission scenario. Without mAb PEP, the estimated cost of hospitalizations due to COVID-19 infections from household exposure in the lower transmission scenario was $149 million (95% UI, $115-$196 million), whereas the estimated hospitalization cost in the higher transmission scenario was $400 million (95% UI, $312-$508 million). In the lower transmission scenario, mAb PEP administered to 50% of eligible contacts aged 80 years and older was estimated to have 82% probability of saving costs, but was not associated with cost savings at age thresholds of 50 years and older or 20 years and older. In contrast, in the high-transmission scenario, mAb PEP administered to 50% of eligible household contacts had estimated cost savings in 100% of simulations at the 80-year age threshold, 96% of simulations at the 50-year threshold, and 2% of simulations at the 20-year thresholds.
Conclusions and relevance: In this modeling study of a simulated US population, a mAb PEP for COVID-19 program was estimated to improve health outcomes and reduce costs. In the setting of a susceptible variant of SARS-CoV-2, health system and public health actors would have an opportunity to improve health and reduce net payer costs through COVID-19 PEP with mAbs.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

