Central memory T cells are the most effective precursors of resident memory T cells in human skin
- PMID: 35452256
- PMCID: PMC9435065
- DOI: 10.1126/sciimmunol.abn1889
Central memory T cells are the most effective precursors of resident memory T cells in human skin
Abstract
The circulating precursor cells that give rise to human resident memory T cells (TRM) are poorly characterized. We used an in vitro differentiation system and human skin-grafted mice to study TRM generation from circulating human memory T cell subsets. In vitro TRM differentiation was associated with functional changes, including enhanced IL-17A production and FOXP3 expression in CD4+ T cells and granzyme B production in CD8+ T cells, changes that mirrored the phenotype of T cells in healthy human skin. Effector memory T cells (TEM) had the highest conversion rate to TRM in vitro and in vivo, but central memory T cells (TCM) persisted longer in the circulation, entered the skin in larger numbers, and generated increased numbers of TRM. In summary, TCM are highly efficient precursors of human skin TRM, a feature that may underlie their known association with effective long-term immunity.
Conflict of interest statement
Figures

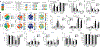



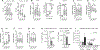
References
-
- Mueller SN, Mackay LK, Tissue-resident memory T cells: local specialists in immune defence. Nature reviews Immunology 16, 79–89 (2016). - PubMed
-
- Sallusto F, Geginat J, Lanzavecchia A, Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annual review of immunology 22, 745–763 (2004). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

