A dominant function of LynB kinase in preventing autoimmunity
- PMID: 35452291
- PMCID: PMC9032976
- DOI: 10.1126/sciadv.abj5227
A dominant function of LynB kinase in preventing autoimmunity
Abstract
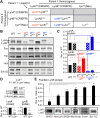
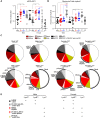
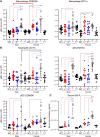
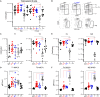
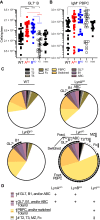
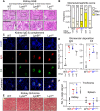
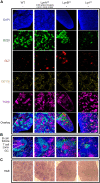
Here, we report that the LynB splice variant of the Src-family kinase Lyn exerts a dominant immunosuppressive function in vivo, whereas the LynA isoform is uniquely required to restrain autoimmunity in female mice. We used CRISPR-Cas9 gene editing to constrain lyn splicing and expression, generating single-isoform LynA knockout (LynAKO) or LynBKO mice. Autoimmune disease in total LynKO mice is characterized by production of antinuclear antibodies, glomerulonephritis, impaired B cell development, and overabundance of activated B cells and proinflammatory myeloid cells. Expression of LynA or LynB alone uncoupled the developmental phenotype from the autoimmune disease: B cell transitional populations were restored, but myeloid cells and differentiated B cells were dysregulated. These changes were isoform-specific, sexually dimorphic, and distinct from the complete LynKO. Despite the apparent differences in disease etiology and penetrance, loss of either LynA or LynB had the potential to induce severe autoimmune disease with parallels to human systemic lupus erythematosus (SLE).
Figures



References
-
- Yamanashi Y., Fukui Y., Wongsasant B., Kinoshita Y., Ichimori Y., Toyoshima K., Yamamoto T., Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc. Natl. Acad. Sci. U.S.A. 89, 1118–1122 (1992). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

