The effectiveness of incentives for research participation: A systematic review and meta-analysis of randomized controlled trials
- PMID: 35452488
- PMCID: PMC9032371
- DOI: 10.1371/journal.pone.0267534
The effectiveness of incentives for research participation: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Recruitment plays a vital role in conducting randomized control trials (RCTs). Challenges and failure of proper recruitment lead to early termination of trials. Monetary incentives have been suggested as a potential solution to these challenges. Therefore, we aimed to do a systematic review and analysis to evaluate the effect of incentives on the number of participants willing to consent to and participate in RCTs.
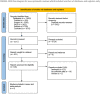
Methods: Electronic databases were systematically searched from inception to September 23rd, 2021, using the following keywords: payments, incentive, response, participation, enrollment, randomized, randomization, and RCT. The Cochrane Risk of Bias tool was used to assess the quality of the included trials. Risk ratios (RRs) were calculated with their corresponding 95% confidence interval (CI). All analyses were done with the random-effects model. We used Revman software to perform the analysis.
Results: Six RCTs with 6,253 Participants met the inclusion criteria. Our analysis showed significant improvement in response rate (RR: 1.27; 95% CI: 1.04, 1.55; P = 0.02) and consent rates (RR: 1.44; 95% CI: 1.11, 1.85; P = 0.006) when an incentive payment was offered to participants. Even a small amount of incentive showed significant improvement in both consent (RR: 1.33; 95% CI: 1.03, 1.73; P = 0.03) and response rates (RR: 1.26; 95% CI: 1.08, 1.47; P = 0.004).
Conclusion: In conclusion, our meta-analysis demonstrated statistically significant increases in the rate of consent and responses from participants when offered even small monetary value incentives. These findings suggest that incentives may be used to reduce the rate of recruitment failure and subsequent study termination. However, further RCTs are needed to establish a critical threshold beyond which incentive amount does not alter response rates further and the types of RCTs in which financial incentives are likely to be effective.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

