The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function
- PMID: 35452600
- PMCID: PMC9116152
- DOI: 10.1016/j.cmet.2022.03.013
The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function
Abstract
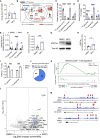
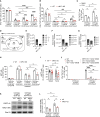
Glycolysis, including both lactate fermentation and pyruvate oxidation, orchestrates CD8+ T cell differentiation. However, how mitochondrial pyruvate metabolism and uptake controlled by the mitochondrial pyruvate carrier (MPC) impact T cell function and fate remains elusive. We found that genetic deletion of MPC drives CD8+ T cell differentiation toward a memory phenotype. Metabolic flexibility induced by MPC inhibition facilitated acetyl-coenzyme-A production by glutamine and fatty acid oxidation that results in enhanced histone acetylation and chromatin accessibility on pro-memory genes. However, in the tumor microenvironment, MPC is essential for sustaining lactate oxidation to support CD8+ T cell antitumor function. We further revealed that chimeric antigen receptor (CAR) T cell manufacturing with an MPC inhibitor imprinted a memory phenotype and demonstrated that infusing MPC inhibitor-conditioned CAR T cells resulted in superior and long-lasting antitumor activity. Altogether, we uncover that mitochondrial pyruvate uptake instructs metabolic flexibility for guiding T cell differentiation and antitumor responses.
Keywords: T cell memory; chimeric antigen receptor T cell therapy; immunometabolism; mitochondrial pyruvate carrier; tumor-infiltrating lymphocyte metabolism.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.W. and P.R. are inventors on a patent application related to these findings and have research collaborations with MPC Therapeutics, with P.R. being a member of the advisory board. D.M. is an inventor on patents related to CAR T cell therapy, filed by the University of Pennsylvania and the University of Geneva, and is a consultant for Limula Therapeutics and MPC Therapeutics. P.-C.H. is scientific advisory for Elixiron Immunotherapeutics, Acepodia, and Novartis. P.-C.H. also receives research support from Elixiron Immunotherapeutics. S.Y.L. is a consultant to Senda Biosciences.
Figures






Comment in
-
Mitochondrial pyruvate carrier: a metabolic-epigenetic checkpoint orchestrating T cell differentiation.Signal Transduct Target Ther. 2022 Aug 12;7(1):280. doi: 10.1038/s41392-022-01101-z. Signal Transduct Target Ther. 2022. PMID: 35961972 Free PMC article. No abstract available.
References
-
- Bourgeois C., Rocha B., Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

