Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia
- PMID: 35452603
- PMCID: PMC9107929
- DOI: 10.1016/j.ccell.2022.04.001
Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia
Abstract
Chimeric antigen receptor (CAR) T cell therapy is effective in lymphoid malignancies, but there has been limited data in myeloid cancers. Here, we start with a CD27-based CAR to target CD70 ("native") in acute myeloid leukemia (AML), and we find modest efficacy in vivo, consistent with prior reports. We then use orthogonal approaches to increase binding on both the tumor and CAR-T cell sides of the immune synapse: a pharmacologic approach (azacitidine) to increase antigen density of CD70 in myeloid tumors, and an engineering approach to stabilize binding of the CAR to CD70. To accomplish the latter, we design a panel of hinge-modified regions to mitigate cleavage of the extracellular portion of CD27. Our CD8 hinge and transmembrane-modified CD70 CAR-T cells are less prone to cleavage, have enhanced binding avidity, and increased expansion, leading to more potent in vivo activity. This enhanced CD70-targeted CAR is a promising candidate for further clinical development.
Keywords: acute myeloid leukemia; adoptive T cell therapy; cell engineering; cellular immunity; chimeric antigen receptors; combined modality therapy; hematologic neoplasms.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and University of Pennsylvania (some licensed to Novartis). M.V.M. holds equity in 2SeventyBio, Century Therapeutics, Genocea, Neximmune, Oncternal, and TCR2 and has served as a consultant for multiple companies involved in cell therapies. M.V.M. is on the Board of Directors of 2Seventy Bio. M.V.M. has grant/research support from CRISPR therapeutics, Kite Pharma, Servier, and Novartis. M.J.F. has served as a consultant for several cell therapy companies. No other authors report conflicts of interest. M.B.L. and M.V.M. have filed patents on the constructs described herein.
Figures
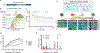

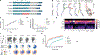



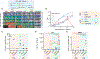
Comment in
-
CD70 CAR T cells in AML: Form follows function.Cell Rep Med. 2022 May 17;3(5):100639. doi: 10.1016/j.xcrm.2022.100639. Cell Rep Med. 2022. PMID: 35584621 Free PMC article.
References
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. (2020). Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet 396, 839–852. 10.1016/S0140-6736(20)31366-0. - DOI - PubMed
-
- Actavis_Pharma, S.P.S. Azacitidine [package insert] U.S. Food and Drug Administration
-
- Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, Yang S, and Kochenderfer JN (2017). Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol Ther 25, 2452–2465. 10.1016/j.ymthe.2017.07.013. - DOI - PMC - PubMed
-
- Boroughs AC, Larson RC, Marjanovic ND, Gosik K, Castano AP, Porter CBM, Lorrey SJ, Ashenberg O, Jerby L, Hofree M, et al. (2020). A Distinct Transcriptional Program in Human CAR T Cells Bearing the 4–1BB Signaling Domain Revealed by scRNA-Seq. Molecular therapy : the journal of the American Society of Gene Therapy 28, 2577–2592. 10.1016/j.ymthe.2020.07.023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

