General anesthesia globally synchronizes activity selectively in layer 5 cortical pyramidal neurons
- PMID: 35452606
- PMCID: PMC9235854
- DOI: 10.1016/j.neuron.2022.03.032
General anesthesia globally synchronizes activity selectively in layer 5 cortical pyramidal neurons
Abstract
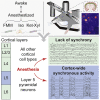
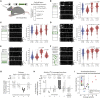
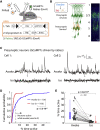
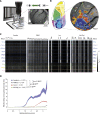
General anesthetics induce loss of consciousness, a global change in behavior. However, a corresponding global change in activity in the context of defined cortical cell types has not been identified. Here, we show that spontaneous activity of mouse layer 5 pyramidal neurons, but of no other cortical cell type, becomes consistently synchronized in vivo by different general anesthetics. This heightened neuronal synchrony is aperiodic, present across large distances, and absent in cortical neurons presynaptic to layer 5 pyramidal neurons. During the transition to and from anesthesia, changes in synchrony in layer 5 coincide with the loss and recovery of consciousness. Activity within both apical and basal dendrites is synchronous, but only basal dendrites' activity is temporally locked to somatic activity. Given that layer 5 is a major cortical output, our results suggest that brain-wide synchrony in layer 5 pyramidal neurons may contribute to the loss of consciousness during general anesthesia.
Keywords: GCaMP7; LOC; ROC; Rbp4-Cre mouse; consciousness; cortex; cortical output; electrophysiology; general anesthesia; layer 5; loss and recovery of consciousness; neuronal activity; pyramidal cells; synchrony; two-photon imaging.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
General anesthesia and the cortical stranglehold on consciousness.Neuron. 2022 Jun 15;110(12):1891-1893. doi: 10.1016/j.neuron.2022.05.014. Neuron. 2022. PMID: 35709695
References
-
- Adams S., Pacharinsak C. Mouse anesthesia and analgesia. Curr. Protoc. Mouse Biol. 2015;5:51–63. - PubMed
-
- Akrawi W.P., Drummond J.C., Kalkman C.J., Patel P.M. A comparison of the electrophysiologic characteristics of EEG burst-suppression as produced by isoflurane, thiopental, etomidate, and propofol. J. Neurosurg. Anesthesiol. 1996;8:40–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

