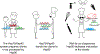There are more Hsp90 chaperone mechanisms in heaven and earth, dear reader, than are dreamt of in your philosophy
- PMID: 35452610
- PMCID: PMC9074108
- DOI: 10.1016/j.molcel.2022.03.040
There are more Hsp90 chaperone mechanisms in heaven and earth, dear reader, than are dreamt of in your philosophy
Abstract
Dahiya et al. (2022) and Biebl et al. (2022) present mechanistic insights into the Hsp40/Hsp70/Hsp90 chaperone teamwork and the co-chaperones that participate in this network.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures
Comment on
-
NudC guides client transfer between the Hsp40/70 and Hsp90 chaperone systems.Mol Cell. 2022 Feb 3;82(3):555-569.e7. doi: 10.1016/j.molcel.2021.12.031. Epub 2022 Jan 20. Mol Cell. 2022. PMID: 35063133
-
The switch from client holding to folding in the Hsp70/Hsp90 chaperone machineries is regulated by a direct interplay between co-chaperones.Mol Cell. 2022 Apr 21;82(8):1543-1556.e6. doi: 10.1016/j.molcel.2022.01.016. Epub 2022 Feb 16. Mol Cell. 2022. PMID: 35176233
References
-
- Dahiya V, Rutz DA, Moessmer P, Mühlhofer M, Lawatscheck J, Rief M, and Buchner J (2022). The switch from client holding to folding in the Hsp70/Hsp90 chaperone machineries is regulated by a direct interplay between co-chaperones. Mol. Cell 82, ■■■. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources