Reshaping endoplasmic reticulum quality control through the unfolded protein response
- PMID: 35452616
- PMCID: PMC9038009
- DOI: 10.1016/j.molcel.2022.03.025
Reshaping endoplasmic reticulum quality control through the unfolded protein response
Abstract
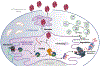
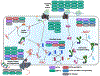
Endoplasmic reticulum quality control (ERQC) pathways comprising chaperones, folding enzymes, and degradation factors ensure the fidelity of ER protein folding and trafficking to downstream secretory environments. However, multiple factors, including tissue-specific secretory proteomes, environmental and genetic insults, and organismal aging, challenge ERQC. Thus, a key question is: how do cells adapt ERQC to match the diverse, ever-changing demands encountered during normal physiology and in disease? The answer lies in the unfolded protein response (UPR), a signaling mechanism activated by ER stress. In mammals, the UPR comprises three signaling pathways regulated downstream of the ER membrane proteins IRE1, ATF6, and PERK. Upon activation, these UPR pathways remodel ERQC to alleviate cellular stress and restore ER function. Here, we describe how UPR signaling pathways adapt ERQC, highlighting their importance for maintaining ER function across tissues and the potential for targeting the UPR to mitigate pathologies associated with protein misfolding diseases.
Keywords: ATF6; ER-associated degradation; ERAD; IRE1; PERK; XBP1s; amyloid; chaperone; loss-of-function disease; protein aggregation; protein misfolding disease.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.L.W. is an inventor on patents for IRE1/XBP1s and ATF6 activating compounds and is a scientific advisory board member and shareholder for Protego Biopharma, which has licensed UPR activating compounds for translational development. No other conflicts are identified.
Figures

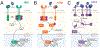
References
-
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, and Mori K (2008). ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct 33, 75–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

