Tigecycline Dosing Strategies in Critically Ill Liver-Impaired Patients
- PMID: 35453230
- PMCID: PMC9028393
- DOI: 10.3390/antibiotics11040479
Tigecycline Dosing Strategies in Critically Ill Liver-Impaired Patients
Abstract
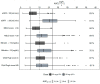
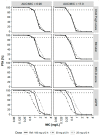
This study investigated tigecycline exposure in critically ill patients from a population pharmacokinetic perspective to support rational dosing in intensive care unit (ICU) patients with acute and chronic liver impairment. A clinical dataset of 39 patients served as the basis for the development of a population pharmacokinetic model. The typical tigecycline clearance was strongly reduced (8.6 L/h) as compared to other populations. Different models were developed based on liver and kidney function-related covariates. Monte Carlo simulations were used to guide dose adjustments with the most predictive covariates: Child-Pugh score, total bilirubin, and MELD score. The best performing covariate, guiding a dose reduction to 25 mg q12h, was Child-Pugh score C, whereas patients with Child-Pugh score A/B received the standard dose of 50 mg q12h. Of note, the obtained 24 h steady-state area under the concentration vs. time curve (AUCss) range using this dosing strategy was predicted to be equivalent to high-dose tigecycline exposure (100 mg q12h) in non-ICU patients. In addition, 26/39 study participants died, and therapy failure was most correlated with chronic liver disease and renal failure, but no correlation between drug exposure and survival was observed. However, tigecycline in special patient populations needs further investigations to enhance clinical outcome.
Keywords: Child–Pugh score; dose adjustment; population pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pfizer Tygacil® Full Prescribing Information. [(accessed on 21 March 2022)]. Available online: https://www.pfizermedicalinformation.com/en-us/tygacil/dosage-admin.
-
- Meagher A.K., Passarell J.A., Cirincione B.B., Van Wart S.A., Liolios K., Babinchak T., Ellis-Grosse E.J., Ambrose P.G. Exposure-Response Analyses of Tigecycline Efficacy in Patients with Complicated Skin and Skin-Structure Infections. Antimicrob. Agents Chemother. 2007;51:1939–1945. doi: 10.1128/AAC.01084-06. - DOI - PMC - PubMed
-
- Passarell J.A., Meagher A.K., Liolios K., Cirincione B.B., Van Wart S.A., Babinchak T., Ellis-Grosse E.J., Ambrose P.G. Exposure-Response Analyses of Tigecycline Efficacy in Patients with Complicated Intra-Abdominal Infections. Antimicrob. Agents Chemother. 2008;52:204–210. doi: 10.1128/AAC.00813-07. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

