Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation
- PMID: 35453302
- PMCID: PMC9027671
- DOI: 10.3390/antiox11040617
Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation
Abstract
Diabetic retinopathy is a neurovascular complication of diabetes and the main cause of vision loss in adults. Glial cells have a key role in maintenance of central nervous system homeostasis. In the retina, the predominant element is the Müller cell, a specialized cell with radial morphology that spans all retinal layers and influences the function of the entire retinal circuitry. Müller cells provide metabolic support, regulation of extracellular composition, synaptic activity control, structural organization of the blood-retina barrier, antioxidant activity, and trophic support, among other roles. Therefore, impairments of Müller actions lead to retinal malfunctions. Accordingly, increasing evidence indicates that Müller cells are affected in diabetic retinopathy and may contribute to the severity of the disease. Here, we will survey recently described alterations in Müller cell functions and cellular events that contribute to diabetic retinopathy, especially related to oxidative stress and inflammation. This review sheds light on Müller cells as potential therapeutic targets of this disease.
Keywords: Müller glia; Nrf2; antioxidants; diabetes; reactive oxidative stress; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

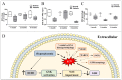
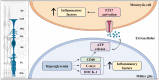
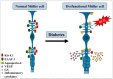

References
-
- Prevention of Diabetes Mellitus . Report of a WHO Study Group. Volume 844 WHO; Geneva, Switzerland: 1994. - PubMed
-
- Pereira-Figueiredo D., Brito R., Araújo D.S.M., Nascimento A.A., Lyra E.S.B., Cheibub A.M.S.S., Pereira Netto A.D., Ventura A.L.M., Paes-de-Carvalho R.C.K., Calaza K.C. Caffeine Exposure Ameliorates Acute Ischemic Cell Death in Avian Developing Retina. Purinergic Signal. 2020;16:41–59. doi: 10.1007/s11302-020-09687-1. - DOI - PMC - PubMed
-
- Hu L., Gong C., Chen X., Zhou H., Yan J., Hong W. Associations between Vascular Endothelial Growth Factor Gene Polymorphisms and Different Types of Diabetic Retinopathy Susceptibility: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2021;2021:7059139. doi: 10.1155/2021/7059139. - DOI - PMC - PubMed
Publication types
Grants and funding
- 304410/2019-5/National Council for Scientific and Technological Development
- 465346/2014-6/Instituto Nacional de Ciência e Tecnologia de Neurociência Translacional
- E-26/200.872/2018/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/201.025/2021/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- 305562/2017-7/National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources

