Reduced Levels of H2S in Diabetes-Associated Osteoarthritis Are Linked to Hyperglycaemia, Nrf-2/HO-1 Signalling Downregulation and Chondrocyte Dysfunction
- PMID: 35453313
- PMCID: PMC9024787
- DOI: 10.3390/antiox11040628
Reduced Levels of H2S in Diabetes-Associated Osteoarthritis Are Linked to Hyperglycaemia, Nrf-2/HO-1 Signalling Downregulation and Chondrocyte Dysfunction
Abstract
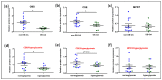
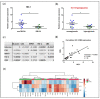
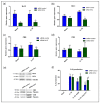
Different findings indicate that type 2 diabetes is an independent risk factor for osteoarthritis (OA). However, the mechanisms underlying the connection between both diseases remain unclear. Changes in the balance of hydrogen sulphide (H2S) are thought to play an important role in the pathogenesis of diabetes and its complications, although its role is still controversial. In this study, we examined the modulation of H2S levels in serum and chondrocytes from OA diabetic (DB) and non-diabetic (non-DB) patients and in cells under glucose stress, in order to elucidate whether impairment in H2S-mediated signalling could participate in the onset of DB-related OA. Here, we identified a reduction in H2S synthesis in the cartilage from OA-DB patients and in cells under glucose stress, which is associated with hyperglycaemia-mediated dysregulation of chondrocyte metabolism. In addition, our results indicate that H2S is an inductor of the Nrf-2/HO-1 signalling pathway in cartilage, but is also a downstream target of Nrf-2 transcriptional activity. Thereby, impairment of the H2S/Nrf-2 axis under glucose stress or DB triggers chondrocyte catabolic responses, favouring the disruption of cartilage homeostasis that characterizes OA pathology. Finally, our findings highlight the benefits of the use of exogeneous sources of H2S in the treatment of DB-OA patients, and warrant future clinical studies.
Keywords: chondrocytes; heme oxygenase-1; hydrogen sulphide; inflammation; nuclear factor-erythroid 2-related factor-2; osteoarthritis; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
The nuclear factor-erythroid 2-related factor/heme oxygenase-1 axis is critical for the inflammatory features of type 2 diabetes-associated osteoarthritis.J Biol Chem. 2017 Sep 1;292(35):14505-14515. doi: 10.1074/jbc.M117.802157. Epub 2017 Jul 6. J Biol Chem. 2017. PMID: 28684418 Free PMC article.
-
Study of hydrogen sulfide biosynthesis in synovial tissue from diabetes-associated osteoarthritis and its influence on macrophage phenotype and abundance.J Physiol Biochem. 2023 Aug;79(3):653-667. doi: 10.1007/s13105-023-00968-y. Epub 2023 Jun 19. J Physiol Biochem. 2023. PMID: 37335394 Free PMC article.
-
The protective role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway against experimental osteoarthritis.Arthritis Res Ther. 2020 Mar 17;22(1):49. doi: 10.1186/s13075-020-02147-6. Arthritis Res Ther. 2020. PMID: 32183900 Free PMC article.
-
Therapeutic potential of hydrogen sulfide in osteoarthritis development.Front Pharmacol. 2024 Feb 2;15:1336693. doi: 10.3389/fphar.2024.1336693. eCollection 2024. Front Pharmacol. 2024. PMID: 38370481 Free PMC article. Review.
-
The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis.Ageing Res Rev. 2021 Mar;66:101249. doi: 10.1016/j.arr.2020.101249. Epub 2020 Dec 29. Ageing Res Rev. 2021. PMID: 33383189 Review.
Cited by
-
In Vitro Study of the Therapeutic Potential of Brown Crude Fucoidans in Osteoarthritis Treatment.Int J Mol Sci. 2022 Nov 17;23(22):14236. doi: 10.3390/ijms232214236. Int J Mol Sci. 2022. PMID: 36430716 Free PMC article.
-
Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells.Antioxidants (Basel). 2023 Feb 8;12(2):417. doi: 10.3390/antiox12020417. Antioxidants (Basel). 2023. PMID: 36829975 Free PMC article.
-
Function of gaseous hydrogen sulfide in liver fibrosis.BMB Rep. 2022 Oct;55(10):481-487. doi: 10.5483/BMBRep.2022.55.10.124. BMB Rep. 2022. PMID: 36195563 Free PMC article. Review.
-
Novel ray of hope for diabetic wound healing: Hydrogen sulfide and its releasing agents.J Adv Res. 2024 Apr;58:105-115. doi: 10.1016/j.jare.2023.05.009. Epub 2023 May 27. J Adv Res. 2024. PMID: 37245638 Free PMC article. Review.
-
Identification and validation of up-regulated TNFAIP6 in osteoarthritis with type 2 diabetes mellitus.Sci Rep. 2024 Dec 28;14(1):31450. doi: 10.1038/s41598-024-82985-5. Sci Rep. 2024. PMID: 39733138 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

