Does Plant Breeding for Antioxidant-Rich Foods Have an Impact on Human Health?
- PMID: 35453479
- PMCID: PMC9024522
- DOI: 10.3390/antiox11040794
Does Plant Breeding for Antioxidant-Rich Foods Have an Impact on Human Health?
Abstract
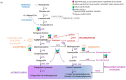
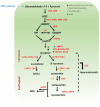
Given the general beneficial effects of antioxidants-rich foods on human health and disease prevention, there is a continuous interest in plant secondary metabolites conferring attractive colors to fruits and grains and responsible, together with others, for nutraceutical properties. Cereals and Solanaceae are important components of the human diet, thus, they are the main targets for functional food development by exploitation of genetic resources and metabolic engineering. In this review, we focus on the impact of antioxidants-rich cereal and Solanaceae derived foods on human health by analyzing natural biodiversity and biotechnological strategies aiming at increasing the antioxidant level of grains and fruits, the impact of agronomic practices and food processing on antioxidant properties combined with a focus on the current state of pre-clinical and clinical studies. Despite the strong evidence in in vitro and animal studies supporting the beneficial effects of antioxidants-rich diets in preventing diseases, clinical studies are still not sufficient to prove the impact of antioxidant rich cereal and Solanaceae derived foods on human.
Keywords: Solanaceae; antioxidants; carotenoids; cereals; food diet; polyphenols; pre-clinical studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Martínez V., Mitjans M., Vinardell M.P. Cytoprotective effects of polyphenols against oxidative damage. In: Watson R.R., Preedy V.R., Sherma Z., editors. Polyphenols in Human Health and Disease. Academic Press; Cambridge, MA, USA: 2014. pp. 275–288.
-
- Marone D., Mastrangelo A.M., Borrelli G.M., Mores A., Laidò G., Russo M.A., Ficco D. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022;172:48–55. doi: 10.1016/j.plaphy.2021.12.037. - DOI - PubMed
-
- FAOSTAT. [(accessed on 3 November 2021)]. Available online: https://www.fao.org/faostat/en/#data.

