The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression
- PMID: 35453557
- PMCID: PMC9031586
- DOI: 10.3390/biomedicines10040806
The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression
Abstract
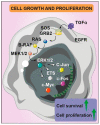
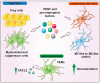
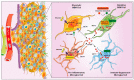
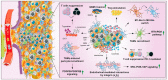
In 2021 the World Health Organization published the fifth and latest version of the Central Nervous System tumors classification, which incorporates and summarizes a long list of updates from the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy work. Among the adult-type diffuse gliomas, glioblastoma represents most primary brain tumors in the neuro-oncology practice of adults. Despite massive efforts in the field of neuro-oncology diagnostics to ensure a proper taxonomy, the identification of glioblastoma-tumor subtypes is not accompanied by personalized therapies, and no improvements in terms of overall survival have been achieved so far, confirming the existence of open and unresolved issues. The aim of this review is to illustrate and elucidate the state of art regarding the foremost biological and molecular mechanisms that guide the beginning and the progression of this cancer, showing the salient features of tumor hallmarks in glioblastoma. Pathophysiology processes are discussed on molecular and cellular levels, highlighting the critical overlaps that are involved into the creation of a complex tumor microenvironment. The description of glioblastoma hallmarks shows how tumoral processes can be linked together, finding their involvement within distinct areas that are engaged for cancer-malignancy establishment and maintenance. The evidence presented provides the promising view that glioblastoma represents interconnected hallmarks that may led to a better understanding of tumor pathophysiology, therefore driving the development of new therapeutic strategies and approaches.
Keywords: glioblastoma; immune modulation; invasiveness; metabolism; stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
- Filippo Torrisi/Fondazione Umberto Veronesi
- Cristiana Alberghina/PhD program in Biotechnology (Biometec, University of Catania, Italy)
- Anna M. Pavone/PhD program in Biotechnology (Biometec, University of Catania, Italy)
- Simona D'Aprile/PhD program in Neuroscience (Biometec, University of Catania, Italy)
- E66C18001240007/PON AIM R&I 2014-2020
LinkOut - more resources
Full Text Sources

