Development of Alzheimer's Disease Biomarkers: From CSF- to Blood-Based Biomarkers
- PMID: 35453600
- PMCID: PMC9025524
- DOI: 10.3390/biomedicines10040850
Development of Alzheimer's Disease Biomarkers: From CSF- to Blood-Based Biomarkers
Abstract
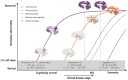
In the 115 years since the discovery of Alzheimer's disease (AD), our knowledge, diagnosis, and therapeutics have significantly improved. Biomarkers are the primary tools for clinical research, diagnostics, and therapeutic monitoring in clinical trials. They provide much insightful information, and while they are not clinically used routinely, they help us to understand the mechanisms of this disease. This review charts the journey of AD biomarker discovery and development from cerebrospinal fluid (CSF) amyloid-beta 1-42 (Aβ42), total tau (T-tau), and phosphorylated tau (p-tau) biomarkers and imaging technologies to the next generation of biomarkers. We also discuss advanced high-sensitivity assay platforms for CSF Aβ42, T-tau, p-tau, and blood analysis. The recently proposed Aβ deposition/tau biomarker/neurodegeneration or neuronal injury (ATN) scheme might facilitate the definition of the biological status underpinning AD and offer a common language among researchers across biochemical biomarkers and imaging. Moreover, we highlight blood-based biomarkers for AD that offer a scalable alternative to CSF biomarkers through cost-saving and reduced invasiveness, and may provide an understanding of disease initiation and development. We discuss different groups of blood-based biomarker candidates, their advantages and limitations, and paths forward, from identification and analysis to clinical validation. The development of valid blood-based biomarkers may facilitate the implementation of future AD therapeutics and diagnostics.
Keywords: Alzheimer’s biomarker; amyloid-beta; blood-based biomarker; cerebrospinal fluid; clinical trial; tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Motter R., Vigo-Pelfrey C., Kholodenko D., Barbour R., Johnson-Wood K., Galasko D., Chang L., Miller B., Clark C., Green R., et al. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. - DOI - PubMed
-
- Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J., Green R.C., Harvey D., Jack C.R., Jagust W., et al. 2014 Update of the Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer’s Dement. 2015;11:e1–e120. doi: 10.1016/j.jalz.2014.11.001. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

