The Impact of Prolonged Inflammation on Wound Healing
- PMID: 35453606
- PMCID: PMC9025535
- DOI: 10.3390/biomedicines10040856
The Impact of Prolonged Inflammation on Wound Healing
Abstract
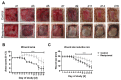
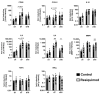
The treatment of chronic wounds still challenges modern medicine because of these wounds' heterogenic pathophysiology. Processes such as inflammation, ischemia and bacterial infection play major roles in the progression of a chronic wound. In recent years, preclinical wound models have been used to understand the underlying processes of chronic wound formation. However, the wound models used to investigate chronic wounds often lack translatability from preclinical models to patients, and often do not take exaggerated inflammation into consideration. Therefore, we aimed to investigate prolonged inflammation in a porcine wound model by using resiquimod, a TLR7 and TLR8 agonist. Pigs received full thickness excisional wounds, where resiquimod was applied daily for 6 days, and untreated wounds served as controls. Dressing change, visual documentation and wound scoring were performed daily. Biopsies were collected for histological as well as gene expression analysis. Resiquimod application on full thickness wounds induced a visible inflammation of wounds, resulting in delayed wound healing compared to non-treated control wounds. Gene expression analysis revealed high levels of IL6, MMP1 and CD68 expression after resiquimod application, and histological analysis showed increased immune cell infiltration. By using resiquimod, we were able to show that prolonged inflammation delayed wound healing, which is often observed in chronic wounds in patients. The model we used shows the importance of inflammation in wound healing and gives an insight into the progression of chronic wounds.
Keywords: inflammation; prolonged inflammation; resiquimod R848; wound healing; wound model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

