COVID-19 Infection Induce miR-371a-3p Upregulation Resulting in Influence on Male Fertility
- PMID: 35453608
- PMCID: PMC9033010
- DOI: 10.3390/biomedicines10040858
COVID-19 Infection Induce miR-371a-3p Upregulation Resulting in Influence on Male Fertility
Abstract
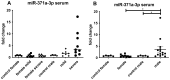
In December 2019, the first case of COVID-19 was reported and since then several groups have already published that the virus can be present in the testis. To study the influence of SARS-CoV-2 which cause a dysregulation of the androgen receptor (AR) level, thereby leading to fertility problems and inducing germ cell testicular changes in patients after the infection. Formalin-Fixed-Paraffin-Embedded (FFPE) testicular samples from patients who died with or as a result of COVID-19 (n = 32) with controls (n = 6), inflammatory changes (n = 9), seminoma with/without metastasis (n = 11) compared with healthy biopsy samples (n = 3) were analyzed and compared via qRT-PCR for the expression of miR-371a-3p. An immunohistochemical analysis (IHC) and ELISA were performed in order to highlight the miR-371a-3p targeting the AR. Serum samples of patients with mild or severe COVID-19 symptoms (n = 34) were analyzed for miR-371a-3p expression. In 70% of the analyzed postmortem testicular tissue samples, a significant upregulation of the miR-371a-3p was detected, and 75% of the samples showed a reduced spermatogenesis. In serum samples, the upregulation of the miR-371a-3p was also detectable. The upregulation of the miR-371a-3p is responsible for the downregulation of the AR in SARS-CoV-2-positive patients, resulting in decreased spermatogenesis. Since the dysregulation of the AR is associated with infertility, further studies have to confirm if the identified dysregulation is regressive after a declining infection.
Keywords: COVID-19; SARS-CoV-2; androgen receptor; male infertility; miR-371a-3p.
Conflict of interest statement
O.A.C. reports grants and personal fees from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, MedPace, Merck/MSD, Pfizer, Scynexis; grants from DFG (German Research Foundation), German Federal Ministry of Research and Education, Immunic, Janssen, Medicines Company, Melinta Therapeutics; personal fees from Allecra Therapeutics, Al-Jazeera Pharmaceuticals, Biocon, Biosys, CoRe Consulting, Entasis, Grupo Biotoscana, IQVIA, Matinas, Menarini, Molecular Partners, MSG-ERC, Mylan, Nabriva, Noxxon, Octapharma, Paratek, PSI, Roche Diagnostics, Seres, Shionogi; others from Wiley (Blackwell); outside the submitted work. P.K. is supported by the German Federal Ministry of Research and Education and the State of North Rhine-Westphalia, Germany and has received non-financial scientific grants from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany, and the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases, University of Cologne, Cologne, Germany, and received lecture honoraria from and/or is advisor to Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, MSD Sharp & Dohme GmbH, Noxxon N.V., Pfizer Pharma GmbH and University Hospital, LMU Munich outside the submitted work.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

