Proteasome Inhibitors Decrease the Viability of Pulmonary Arterial Smooth Muscle Cells by Restoring Mitofusin-2 Expression under Hypoxic Conditions
- PMID: 35453623
- PMCID: PMC9030547
- DOI: 10.3390/biomedicines10040873
Proteasome Inhibitors Decrease the Viability of Pulmonary Arterial Smooth Muscle Cells by Restoring Mitofusin-2 Expression under Hypoxic Conditions
Abstract
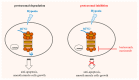
Pulmonary hypertension (PH) is a severe progressive disease, and the uncontrolled proliferation of pulmonary artery smooth muscle cells (PASMCs) is one of the main causes. Mitofusin-2 (MFN2) profoundly inhibits cell growth and proliferation in a variety of tumor cell lines and rat vascular smooth muscle cells. Down-regulation of MFN2 is known to contribute to PH. Proteasome inhibitors have been shown to inhibit the proliferation of PASMCs; however, there is no study on the regulation of proteasome inhibitors through MFN-2 in the proliferation of PASMCs, a main pathophysiology of PH. In this study, PASMCs were exposed to hypoxic conditions and the expression of MFN2 and cleaved-PARP1 were detected by Western blotting. The effects of hypoxia and proteasome inhibitors on the cell viability of PASMC cells were detected by CCK8 assay. The results indicated that hypoxia increases the viability and reduces the expression of MFN2 in a PASMCs model. MFN2 overexpression inhibits the hypoxia-induced proliferation of PASMCs. In addition, proteasome inhibitors, bortezomib and marizomib, restored the decreased expression of MFN2 under hypoxic conditions, inhibited hypoxia-induced proliferation and induced the expression of cleaved-PARP1. These results suggest that bortezomib and marizomib have the potential to improve the hypoxia-induced proliferation of PASMCs by restoring MFN2 expression.
Keywords: hypoxia; mitofusin-2; proteasome inhibitor; pulmonary hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



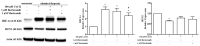
References
-
- Pullamsetti S.S., Savai R., Seeger W., Goncharova E.A. Translational Advances in the Field of Pulmonary Hypertension. From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. Am. J. Respir. Crit. Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

