New Approaches to Diabetic Nephropathy from Bed to Bench
- PMID: 35453626
- PMCID: PMC9031931
- DOI: 10.3390/biomedicines10040876
New Approaches to Diabetic Nephropathy from Bed to Bench
Abstract
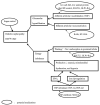
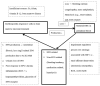
Diabetic nephropathy (DN) is the main cause of end-stage kidney disease (ESKD). DN-related ESKD has the worst prognosis for survival compared with other causes. Due to the complex mechanisms of DN and the heterogeneous presentations, unmet needs exist for the renal outcome of diabetes mellitus. Clinical evidence for treating DN is rather solid. For example, the first Kidney Disease: Improving Global Outcomes (KDIGO) guideline was published in October 2020: KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. In December of 2020, the International Society of Nephrology published 60 (+1) breakthrough discoveries in nephrology. Among these breakthroughs, four important ones after 1980 were recognized, including glomerular hyperfiltration theory, renal protection by renin-angiotensin system inhibition, hypoxia-inducible factor, and sodium-glucose cotransporter 2 inhibitors. Here, we present a review on the pivotal and new mechanisms of DN from the implications of clinical studies and medications.
Keywords: anemia; chronic kidney disease; diabetic nephropathy; glomerular hyperfiltration; hypoxia; hypoxia-inducible factor (HIF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tricco A.C., Ivers N.M., Grimshaw J.M., Moher D., Turner L., Galipeau J., Halperin I., Vachon B., Ramsay T., Manns B., et al. Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta-analysis. Lancet. 2012;379:2252–2261. doi: 10.1016/S0140-6736(12)60480-2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

