Investigating the Effects of Conditioned Media from Stem Cells of Human Exfoliated Deciduous Teeth on Dental Pulp Stem Cells
- PMID: 35453661
- PMCID: PMC9027398
- DOI: 10.3390/biomedicines10040906
Investigating the Effects of Conditioned Media from Stem Cells of Human Exfoliated Deciduous Teeth on Dental Pulp Stem Cells
Abstract
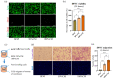
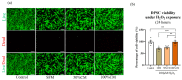
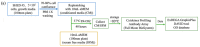
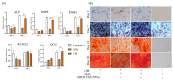
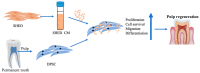
Pulp regeneration has recently attracted interest in modern dentistry. However, the success ratio of pulp regeneration is low due to the compromising potential of stem cells, such as their survival, migration, and odontoblastic differentiation. Stem cells from human exfoliated deciduous teeth (SHED) have been considered a promising tool for regenerative therapy due to their ability to secrete multiple factors that are essential for tissue regeneration, which is achieved by minimally invasive procedures with fewer ethical or legal concerns than those of other procedures. The aim of this study is to investigate the potency of SHED-derived conditioned media (SHED CM) on dental pulp stem cells (DPSCs), a major type of mesenchymal stem cells for dental pulp regeneration. Our results show the promotive efficiency of SHED CM on the proliferation, survival rate, and migration of DPSCs in a dose-dependent manner. Upregulation of odontoblast/osteogenic-related marker genes, such as ALP, DSPP, DMP1, OCN, and RUNX2, and enhanced mineral deposition of impaired DPSCs are also observed in the presence of SHED CM. The analysis of SHED CM found that a variety of cytokines and growth factors have positive effects on cell proliferation, migration, anti-apoptosis, and odontoblast/osteogenic differentiation. These findings suggest that SHED CM could provide some benefits to DPSCs in pulp regeneration.
Keywords: conditioned medium; cytokines; dental pulp stem cell; human exfoliated deciduous teeth; odontoblast/osteogenic differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

