Evaluation of the Cardiometabolic Disorders after Spinal Cord Injury in Mice
- PMID: 35453695
- PMCID: PMC9027794
- DOI: 10.3390/biology11040495
Evaluation of the Cardiometabolic Disorders after Spinal Cord Injury in Mice
Abstract
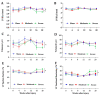
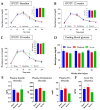
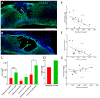
Changes in cardiometabolic functions contribute to increased morbidity and mortality after chronic spinal cord injury. Despite many advancements in discovering SCI-induced pathologies, the cardiometabolic risks and divergences in severity-related responses have yet to be elucidated. Here, we examined the effects of SCI severity on functional recovery and cardiometabolic functions following moderate (50 kdyn) and severe (75 kdyn) contusions in the thoracic-8 (T8) vertebrae in mice using imaging, morphometric, and molecular analyses. Both severities reduced hindlimbs motor functions, body weight (g), and total body fat (%) at all-time points up to 20 weeks post-injury (PI), while only severe SCI reduced the total body lean (%). Severe SCI increased liver echogenicity starting from 12 weeks PI, with an increase in liver fibrosis in both moderate and severe SCI. Severe SCI mice showed a significant reduction in left ventricular internal diameters and LV volume at 20 weeks PI, associated with increased LV ejection fraction as well as cardiac fibrosis. These cardiometabolic dysfunctions were accompanied by changes in the inflammation profile, varying with the severity of the injury, but not in the lipid profile nor cardiac or hepatic tyrosine hydroxylase innervation changes, suggesting that systemic inflammation may be involved in these SCI-induced health complications.
Keywords: cardiometabolic disease; fibrosis; liver and cardiac dysfunctions; spinal cord injury severity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Spinal cord injury-induced cardiomyocyte atrophy and impaired cardiac function are severity dependent.Exp Physiol. 2018 Feb 1;103(2):179-189. doi: 10.1113/EP086549. Epub 2018 Jan 9. Exp Physiol. 2018. PMID: 29235182
-
Spatiotemporal CCR1, CCL3(MIP-1α), CXCR4, CXCL12(SDF-1α) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain.J Neurosurg Spine. 2011 May;14(5):583-97. doi: 10.3171/2010.12.SPINE10480. Epub 2011 Feb 18. J Neurosurg Spine. 2011. PMID: 21332278
-
Angiotensin-II receptor type Ia does not contribute to cardiac atrophy following high-thoracic spinal cord injury in mice.Exp Physiol. 2020 Aug;105(8):1316-1325. doi: 10.1113/EP088378. Epub 2020 Jun 19. Exp Physiol. 2020. PMID: 32515106
-
Role of exercise on visceral adiposity after spinal cord injury: a cardiometabolic risk factor.Eur J Appl Physiol. 2021 Aug;121(8):2143-2163. doi: 10.1007/s00421-021-04688-3. Epub 2021 Apr 23. Eur J Appl Physiol. 2021. PMID: 33891156 Review.
-
Anthropometric Prediction of Visceral Adiposity in Persons With Spinal Cord Injury.Top Spinal Cord Inj Rehabil. 2021;27(1):23-35. doi: 10.46292/sci20-00055. Top Spinal Cord Inj Rehabil. 2021. PMID: 33814881 Free PMC article. Review.
Cited by
-
Neuroanatomical and neurochemical atlas of the spiny mouse (Acomys cahirinus) spinal cord.Brain Struct Funct. 2025 Jul 28;230(7):124. doi: 10.1007/s00429-025-02982-w. Brain Struct Funct. 2025. PMID: 40719809
-
Spinal cord injury-induced metabolic impairment and steatohepatitis develops in non-obese rats and is exacerbated by premorbid obesity.Exp Neurol. 2024 Sep;379:114847. doi: 10.1016/j.expneurol.2024.114847. Epub 2024 Jun 8. Exp Neurol. 2024. PMID: 38852834 Free PMC article.
-
Aryl hydrocarbon receptor restricts axon regeneration of DRG neurons in response to injury.bioRxiv [Preprint]. 2024 Sep 14:2023.11.04.565649. doi: 10.1101/2023.11.04.565649. bioRxiv. 2024. PMID: 37961567 Free PMC article. Preprint.
-
Impact of commonly administered drugs on the progression of spinal cord injury: a systematic review.Commun Med (Lond). 2024 Oct 24;4(1):213. doi: 10.1038/s43856-024-00638-0. Commun Med (Lond). 2024. PMID: 39448737 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

