Preliminary Studies on the Effects of Oyster Mushroom Spherical Virus China Strain on the Mycelial Growth and Fruiting Body Yield of the Edible Mushroom Pleurotus ostreatus
- PMID: 35453773
- PMCID: PMC9029326
- DOI: 10.3390/biology11040574
Preliminary Studies on the Effects of Oyster Mushroom Spherical Virus China Strain on the Mycelial Growth and Fruiting Body Yield of the Edible Mushroom Pleurotus ostreatus
Abstract
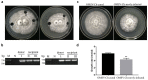
Oyster mushroom spherical virus (OMSV) is a positive-sense single-stranded RNA mycovirus which is associated with a devastating oyster mushroom die-back disease. However, little is known about its diversity, and the effects of OMSV infection on its fungal host are not well understood. In this study, we determined the nearly complete nucleotide sequence of OMSV isolated from cultivated oyster mushrooms in China. Sequence analysis suggested that the virus represents a new strain of OMSV (referred to here as OMSV-Ch). A GenBank BLAST search of the genomic sequences demonstrated that the OMSV-Ch had the highest identity (74.9%) with the OMSV from Korea (OMSV-Kr). At the amino acid-sequence level, these two strains shared 84.1% identity in putative replication protein (RP) and 94.1% identity in coat protein (CP). Phylogenetic analysis based on RP showed that OMSV-Ch clustered with OMSV-Kr, closely related to Tymoviridae. Phylogenetic analysis based on both the RP and CP showed that OMSV had a distant clade relationship with tymoviruses, marafiviruses, and maculaviruses. We obtained the OMSV-Ch-free Pleurotus ostreatus strain via single hyphal tip cultures combined with high-temperature treatment. Preliminary studies indicate that OMSV-Ch can significantly inhibit mycelial growth, cause malformations of the fruiting bodies, and reduce the yield of P. ostreatus. Co-cultivation resulted in horizontal transmission of the OMSV-Ch to a virus-cured strain. The findings of our study contribute to the prevention and control of mycoviral diseases in the future.
Keywords: Pleurotus ostreatus; genome sequence; oyster mushroom spherical virus; phylogenetic analysis; virus curing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Fuke K., Takeshita K., Aoki N., Fukuhara T., Egusa M., Kodama M., Moriyama H. The presence of double-stranded RNAs in Alternaria alternata Japanese pear pathotype is associated with morphological changes. J. Gen. Plant Pathol. 2011;77:248–252. doi: 10.1007/s10327-011-0315-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

