Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body
- PMID: 35454090
- PMCID: PMC9025450
- DOI: 10.3390/biom12040501
Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body
Abstract
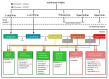
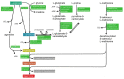
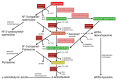
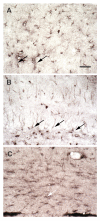
Polyamines (PAs) are small, versatile molecules with two or more nitrogen-containing positively charged groups and provide widespread biological functions. Most of these aspects are well known and covered by quite a number of excellent surveys. Here, the present review includes novel aspects and questions: (1) It summarizes the role of most natural and some important synthetic PAs. (2) It depicts PA uptake from nutrition and bacterial production in the intestinal system following loss of PAs via defecation. (3) It highlights the discrepancy between the high concentrations of PAs in the gut lumen and their low concentration in the blood plasma and cerebrospinal fluid, while concentrations in cellular cytoplasm are much higher. (4) The present review provides a novel and complete scheme for the biosynthesis of Pas, including glycine, glutamate, proline and others as PA precursors, and provides a hypothesis that the agmatine pathway may rescue putrescine production when ODC knockout seems to be lethal (solving the apparent contradiction in the literature). (5) It summarizes novel data on PA transport in brain glial cells explaining why these cells but not neurons preferentially accumulate PAs. (6) Finally, it provides a novel and complete scheme for PA interconversion, including hypusine, putreanine, and GABA (unique gliotransmitter) as end-products. Altogether, this review can serve as an updated contribution to understanding the PA mystery.
Keywords: CNS; agmatine; astrocytes; glial cells; neurons; nutrition; polyamines; spermidine; spermine; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

