Curcumin and Ethanol Effects in Trembler-J Schwann Cell Culture
- PMID: 35454103
- PMCID: PMC9025918
- DOI: 10.3390/biom12040515
Curcumin and Ethanol Effects in Trembler-J Schwann Cell Culture
Abstract
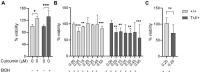
Charcot-Marie-Tooth (CMT) syndrome is the most common progressive human motor and sensory peripheral neuropathy. CMT type 1E is a demyelinating neuropathy affecting Schwann cells due to peripheral-myelin-protein-22 (PMP22) mutations, modelized by Trembler-J mice. Curcumin, a natural polyphenol compound obtained from turmeric (Curcuma longa), exhibits dose- and time-varying antitumor, antioxidant and neuroprotective properties, however, the neurotherapeutic actions of curcumin remain elusive. Here, we propose curcumin as a possible natural treatment capable of enhancing cellular detoxification mechanisms, resulting in an improvement of the neurodegenerative Trembler-J phenotype. Using a refined method for obtaining enriched Schwann cell cultures, we evaluated the neurotherapeutic action of low dose curcumin treatment on the PMP22 expression, and on the chaperones and autophagy/mammalian target of rapamycin (mTOR) pathways in Trembler-J and wild-type genotypes. In wild-type Schwann cells, the action of curcumin resulted in strong stimulation of the chaperone and macroautophagy pathway, whereas the modulation of ribophagy showed a mild effect. However, despite the promising neuroprotective effects for the treatment of neurological diseases, we demonstrate that the action of curcumin in Trembler-J Schwann cells could be impaired due to the irreversible impact of ethanol used as a common curcumin vehicle necessary for administration. These results contribute to expanding our still limited understanding of PMP22 biology in neurobiology and expose the intrinsic lability of the neurodegenerative Trembler-J genotype. Furthermore, they unravel interesting physiological mechanisms of cellular resilience relevant to the pharmacological treatment of the neurodegenerative Tremble J phenotype with curcumin and ethanol. We conclude that the analysis of the effects of the vehicle itself is an essential and inescapable step to comprehensibly assess the effects and full potential of curcumin treatment for therapeutic purposes.
Keywords: CMT1E; Hsps; Trembler-J; autophagy; curcumin; ethanol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Central Alteration in Peripheral Neuropathy of Trembler-J Mice: Hippocampal pmp22 Expression and Behavioral Profile in Anxiety Tests.Biomolecules. 2021 Apr 19;11(4):601. doi: 10.3390/biom11040601. Biomolecules. 2021. PMID: 33921657 Free PMC article.
-
Inducible HSP70 is critical in preventing the aggregation and enhancing the processing of PMP22.ASN Neuro. 2015 Feb 18;7(1):1759091415569909. doi: 10.1177/1759091415569909. Print 2015 Jan-Feb. ASN Neuro. 2015. PMID: 25694550 Free PMC article.
-
Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy.Am J Hum Genet. 2007 Sep;81(3):438-53. doi: 10.1086/519926. Epub 2007 Aug 3. Am J Hum Genet. 2007. PMID: 17701891 Free PMC article.
-
[Hereditary neuropathy: recent advance].Rinsho Shinkeigaku. 2008 Nov;48(11):1019-22. doi: 10.5692/clinicalneurol.48.1019. Rinsho Shinkeigaku. 2008. PMID: 19198150 Review. Japanese.
-
Rodent models with expression of PMP22: Relevance to dysmyelinating CMT and HNPP.J Neurol Sci. 2019 Mar 15;398:79-90. doi: 10.1016/j.jns.2019.01.030. Epub 2019 Jan 21. J Neurol Sci. 2019. PMID: 30685714 Review.
Cited by
-
From in vivo models to in vitro bioengineered neuromuscular junctions for the study of Charcot-Marie-Tooth disease.J Tissue Eng. 2025 Mar 12;16:20417314241310508. doi: 10.1177/20417314241310508. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 40078221 Free PMC article. Review.
-
In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice.Biology (Basel). 2023 Oct 10;12(10):1324. doi: 10.3390/biology12101324. Biology (Basel). 2023. PMID: 37887034 Free PMC article.
-
A low dose of curcumin-PDA nanoparticles improves viability and proliferation in endoneurial fibroblasts and Schwann cell cultures.Discov Nano. 2024 May 7;19(1):81. doi: 10.1186/s11671-024-04023-7. Discov Nano. 2024. PMID: 38714630 Free PMC article.
-
Evaluation and Comparison of the Effects of Mature Silkworm (Bombyx mori) and Silkworm Pupae Extracts on Schwann Cell Proliferation and Axon Growth: An In Vitro Study.Iran J Pharm Res. 2023 Feb 21;21(1):e133552. doi: 10.5812/ijpr-133552. eCollection 2022 Dec. Iran J Pharm Res. 2023. PMID: 36896320 Free PMC article.
References
-
- Parmantier E., Braun C., Thomas J.L., Peyron F., Martinez S., Zalc B. PMP-22 expression in the central nervous system of the embryonic mouse defines potential transverse segments and longitudinal columns. J. Comp. Neurol. 1997;378:159–172. doi: 10.1002/(SICI)1096-9861(19970210)378:2<159::AID-CNE1>3.0.CO;2-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

