Equine Mesenchymal Stem Cells Influence the Proliferative Response of Lymphocytes: Effect of Inflammation, Differentiation and MHC-Compatibility
- PMID: 35454231
- PMCID: PMC9031781
- DOI: 10.3390/ani12080984
Equine Mesenchymal Stem Cells Influence the Proliferative Response of Lymphocytes: Effect of Inflammation, Differentiation and MHC-Compatibility
Abstract
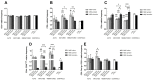
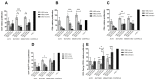
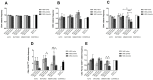
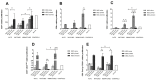
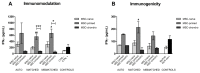
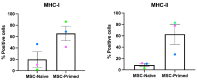
Immunomodulation and immunogenicity are pivotal aspects for the therapeutic use of mesenchymal stem cells (MSCs). Since the horse is highly valuable as both a patient and translational model, further knowledge on equine MSC immune properties is required. This study analysed how inflammation, chondrogenic differentiation and compatibility for the major histocompatibility complex (MHC) influence the MSC immunomodulatory-immunogenicity balance. Equine MSCs in basal conditions, pro-inflammatory primed (MSC-primed) or chondrogenically differentiated (MSC-chondro) were co-cultured with either autologous or allogeneic MHC-matched/mismatched lymphocytes in immune-suppressive assays (immunomodulation) and in modified one-way mixed leukocyte reactions (immunogenicity). After co-culture, frequency and proliferation of T cell subsets and B cells were assessed by flow cytometry and interferon-ɣ (IFNɣ) secretion by ELISA. MSC-primed showed higher regulatory potential by decreasing proliferation of cytotoxic and helper T cells and B cells. However, MHC-mismatched MSC-primed can also activate lymphocytes (proliferative response and IFNɣ secretion), likely due to increased MHC-expression. MSC-chondro maintained their regulatory ability and did not increase their immunogenicity, but showed less capacity than MSC-primed to induce regulatory T cells and further stimulated B cells. Subsequent in vivo studies are needed to elucidate the complex interactions between MSCs and the recipient immune system, which is critical to develop safe and effective therapies.
Keywords: allogenic therapy; co-culture; flow cytometry; haplotype; horse; immunogenicity; immunomodulation; immunosuppression assay; mesenchymal stem cells; modified mixed leukocyte reaction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Wang S., Zhao R.C. A historical overview and concepts of mesenchymal stem cells. In: Zhao R.C., editor. Essentials of Mesenchymal Stem Cell Biology and Its Clinical Translation. Springer; Heidelberg, Germany: 2013. pp. 3–15.
-
- Lo Monaco M., Merckx G., Ratajczak J., Gervois P., Hilkens P., Clegg P., Bronckaers A., Vandeweerd J.M., Lambrichts I. Stem cells for cartilage repair: Preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018;2018:22. doi: 10.1155/2018/9079538. - DOI - PMC - PubMed
-
- Ribitsch I., Baptista P.M., Lange-Consiglio A., Melotti L., Patruno M., Jenner F., Schnabl-Feichter E., Dutton L.C., Connolly D.J., van Steenbeek F.G., et al. Large animal models in regenerative medicine and tissue engineering: To do or not to do. Front. Bioeng. Biotechnol. 2020;8:972. doi: 10.3389/fbioe.2020.00972. - DOI - PMC - PubMed
-
- Hillmann A., Paebst F., Brehm W., Piehler D., Schubert S., Tárnok A., Burk J. A novel direct co-culture assay analyzed by multicolor flow cytometry reveals context-and cell type-specific immunomodulatory effects of equine mesenchymal stromal cells. PLoS ONE. 2019;14:e0218949. doi: 10.1371/journal.pone.0218949. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

