Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation
- PMID: 35454500
- PMCID: PMC9031922
- DOI: 10.3390/ma15082807
Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation
Abstract
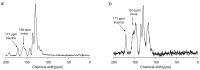
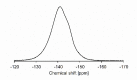
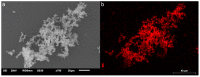
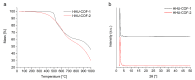
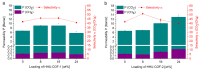
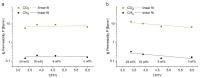
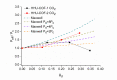
A catalyst-free Schiff base reaction was applied to synthesize two imine-linked covalent organic frameworks (COFs). The condensation reaction of 1,3,5-tris-(4-aminophenyl)triazine (TAPT) with 4,4'-biphenyldicarboxaldehyde led to the structure of HHU-COF-1 (HHU = Heinrich-Heine University). The fluorinated analog HHU-COF-2 was obtained with 2,2',3,3',5,5',6,6'-octafluoro-4,4'-biphenyldicarboxaldehyde. Solid-state NMR, infrared spectroscopy, X-ray photoelectron spectroscopy, and elemental analysis confirmed the successful formation of the two network structures. The crystalline materials are characterized by high Brunauer-Emmett-Teller surface areas of 2352 m2/g for HHU-COF-1 and 1356 m2/g for HHU-COF-2. The products of a larger-scale synthesis were applied to prepare mixed-matrix membranes (MMMs) with the polymer Matrimid. CO2/CH4 permeation tests revealed a moderate increase in CO2 permeability at constant selectivity for HHU-COF-1 as a dispersed phase, whereas application of the fluorinated COF led to a CO2/CH4 selectivity increase from 42 for the pure Matrimid membrane to 51 for 8 wt% of HHU-COF-2 and a permeability increase from 6.8 to 13.0 Barrer for the 24 wt% MMM.
Keywords: CO2/CH4 separation; covalent organic framework (COF); fluorinated COF; imine-COF; mixed-matrix membrane (MMM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

