MBP-11901 Inhibits Tumor Growth of Hepatocellular Carcinoma through Multitargeted Inhibition of Receptor Tyrosine Kinases
- PMID: 35454900
- PMCID: PMC9030223
- DOI: 10.3390/cancers14081994
MBP-11901 Inhibits Tumor Growth of Hepatocellular Carcinoma through Multitargeted Inhibition of Receptor Tyrosine Kinases
Abstract
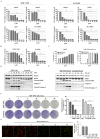
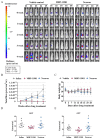
Hepatocellular carcinomas (HCCs) are aggressive tumors with a poor prognosis. Approved first-line treatments include sorafenib, lenvatinib, and a combination of atezolizumab and bevacizumab; however, they do not cure HCC. We investigated MBP-11901 as a drug candidate for HCC. Cell proliferation and cytotoxicity were evaluated using normal and cancer human liver cell lines, while Western blotting and flow cytometry evaluated apoptosis. The anticancer effect of MBP-11901 was verified in vitro through migration, invasion, colony formation, and JC-1 MMP assays. In mouse models, the tumor volume, tumor weight, and bodyweight were measured, and cancer cell proliferation and apoptosis were analyzed. The toxicity of MBP-11901 was investigated through GOT/GPT and histological analyses in the liver and kidney. The signaling mechanism of MBP-11901 was investigated through kinase assays, phosphorylation analysis, and in silico docking simulations. Results. MBP-11901 was effective against various human HCC cell lines, leading to the disappearance of most tumors when administered orally in animal models. This effect was dose-dependent, with no differences in efficacy according to administration intervals. MBP-11901 induced anticancer effects by targeting the signaling mechanisms of FLT3, VEGFR2, c-KIT, and PDGFRβ. MBP-11901 is suggested as a novel therapeutic agent for the treatment of advanced or unresectable liver cancer.
Keywords: anticancer agent; complete response; hepatocellular carcinoma; targeted therapy; tyrosine kinase inhibitor.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures








References
-
- Beaver J.A., Howie L.J., Pelosof L., Kim T., Liu J., Goldberg K.B., Sridhara R., Blumenthal G.M., Farrell A.T., Keegan P. A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: A review. JAMA Oncol. 2018;4:849–856. doi: 10.1001/jamaoncol.2017.5618. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

