Development and Functional Characterization of a Versatile Radio-/Immunotheranostic Tool for Prostate Cancer Management
- PMID: 35454902
- PMCID: PMC9027777
- DOI: 10.3390/cancers14081996
Development and Functional Characterization of a Versatile Radio-/Immunotheranostic Tool for Prostate Cancer Management
Abstract
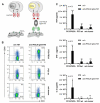
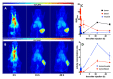
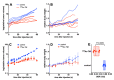
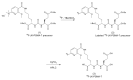
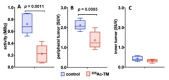
Due to its overexpression on the surface of prostate cancer (PCa) cells, the prostate stem cell antigen (PSCA) is a potential target for PCa diagnosis and therapy. Here we describe the development and functional characterization of a novel IgG4-based anti-PSCA antibody (Ab) derivative (anti-PSCA IgG4-TM) that is conjugated with the chelator DOTAGA. The anti-PSCA IgG4-TM represents a multimodal immunotheranostic compound that can be used (i) as a target module (TM) for UniCAR T cell-based immunotherapy, (ii) for diagnostic positron emission tomography (PET) imaging, and (iii) targeted alpha therapy. Cross-linkage of UniCAR T cells and PSCA-positive tumor cells via the anti-PSCA IgG4-TM results in efficient tumor cell lysis both in vitro and in vivo. After radiolabeling with 64Cu2+, the anti-PSCA IgG4-TM was successfully applied for high contrast PET imaging. In a PCa mouse model, it showed specific accumulation in PSCA-expressing tumors, while no uptake in other organs was observed. Additionally, the DOTAGA-conjugated anti-PSCA IgG4-TM was radiolabeled with 225Ac3+ and applied for targeted alpha therapy. A single injection of the 225Ac-labeled anti-PSCA IgG4-TM was able to significantly control tumor growth in experimental mice. Overall, the novel anti-PSCA IgG4-TM represents an attractive first member of a novel group of radio-/immunotheranostics that allows diagnostic imaging, endoradiotherapy, and CAR T cell immunotherapy.
Keywords: (18)F-JK-PSMA-7; Ac-225; CAR T cell; Cu-64; IgG4; PSCA; PSMA; prostate cancer; theranostics.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





References
-
- Farolfi A., Hadaschik B., Hamdy F.C., Herrmann K., Hofman M.S., Murphy D.G., Ost P., Padhani A.R., Fanti S. Positron Emission Tomography and Whole-body Magnetic Resonance Imaging for Metastasis-directed Therapy in Hormone-sensitive Oligometastatic Prostate Cancer After Primary Radical Treatment: A Systematic Review. Eur. Urol. Oncol. 2021;4:714–730. doi: 10.1016/j.euo.2021.02.003. - DOI - PubMed
-
- FDA Approves First PSMA-Targeted PET Drug. [(accessed on 5 March 2022)];J. Nucl. Med. 2021 62:11N. Available online: https://pubmed.ncbi.nlm.nih.gov/33468545/ - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

