T-Cells Expressing a Highly Potent PRAME-Specific T-Cell Receptor in Combination with a Chimeric PD1-41BB Co-Stimulatory Receptor Show a Favorable Preclinical Safety Profile and Strong Anti-Tumor Reactivity
- PMID: 35454906
- PMCID: PMC9030144
- DOI: 10.3390/cancers14081998
T-Cells Expressing a Highly Potent PRAME-Specific T-Cell Receptor in Combination with a Chimeric PD1-41BB Co-Stimulatory Receptor Show a Favorable Preclinical Safety Profile and Strong Anti-Tumor Reactivity
Abstract
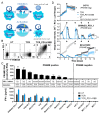
The hostile tumor microenvironment (TME) is a major challenge for the treatment of solid tumors with T-cell receptor (TCR)-modified T-cells (TCR-Ts), as it negatively influences T-cell efficacy, fitness, and persistence. These negative influences are caused, among others, by the inhibitory checkpoint PD-1/PD-L1 axis. The Preferentially Expressed Antigen in Melanoma (PRAME) is a highly relevant cancer/testis antigen for TCR-T immunotherapy due to broad expression in multiple solid cancer indications. A TCR with high specificity and sensitivity for PRAME was isolated from non-tolerized T-cell repertoires and introduced into T-cells alongside a chimeric PD1-41BB receptor, consisting of the natural extracellular domain of PD-1 and the intracellular signaling domain of 4-1BB, turning an inhibitory pathway into a T-cell co-stimulatory pathway. The addition of PD1-41BB to CD8+ T-cells expressing the transgenic PRAME-TCR enhanced IFN-γ secretion, improved cytotoxic capacity, and prevented exhaustion upon repetitive re-challenge with tumor cells in vitro without altering the in vitro safety profile. Furthermore, a single dose of TCR-Ts co-expressing PD1-41BB was sufficient to clear a hard-to-treat melanoma xenograft in a mouse model, whereas TCR-Ts without PD1-41BB could not eradicate the PD-L1-positive tumors. This cutting-edge strategy supports development efforts to provide more effective TCR-T immunotherapies for the treatment of solid tumors.
Keywords: PD-1; PRAME; TCR-T cells; TME; cancer; immunotherapy.
Conflict of interest statement
M.S., D.B., C.G., K.M., and M.B. (Maja Bürdek) are employees and D.J.S. is a Managing Director of Medigene Immunotherapies GmbH, a subsidiary of Medigene AG, Planegg, Germany. N.S., I.F., C.K., M.B. (Monika Braun), S.W., and D.S. were employees of Medigene Immunotherapies GmbH during their contributions to this publication. N.S., M.B. (Monika Braun), and D.S. are current employees at Evotec; S.W. is a current employee at SCG Cell Therapy GmbH; I.F. is a current employee at Juno Therapeutics GmbH, a Bristol Myers Squibb Company; C.K. is a current employee at Intercept Pharma Deutschland GmbH.
Figures



References
-
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

