Deep-Tissue Activation of Photonanomedicines: An Update and Clinical Perspectives
- PMID: 35454910
- PMCID: PMC9032169
- DOI: 10.3390/cancers14082004
Deep-Tissue Activation of Photonanomedicines: An Update and Clinical Perspectives
Abstract
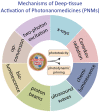
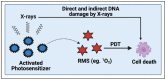
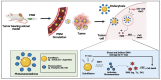
With the continued development of nanomaterials over the past two decades, specialized photonanomedicines (light-activable nanomedicines, PNMs) have evolved to become excitable by alternative energy sources that typically penetrate tissue deeper than visible light. These sources include electromagnetic radiation lying outside the visible near-infrared spectrum, high energy particles, and acoustic waves, amongst others. Various direct activation mechanisms have leveraged unique facets of specialized nanomaterials, such as upconversion, scintillation, and radiosensitization, as well as several others, in order to activate PNMs. Other indirect activation mechanisms have leveraged the effect of the interaction of deeply penetrating energy sources with tissue in order to activate proximal PNMs. These indirect mechanisms include sonoluminescence and Cerenkov radiation. Such direct and indirect deep-tissue activation has been explored extensively in the preclinical setting to facilitate deep-tissue anticancer photodynamic therapy (PDT); however, clinical translation of these approaches is yet to be explored. This review provides a summary of the state of the art in deep-tissue excitation of PNMs and explores the translatability of such excitation mechanisms towards their clinical adoption. A special emphasis is placed on how current clinical instrumentation can be repurposed to achieve deep-tissue PDT with the mechanisms discussed in this review, thereby further expediting the translation of these highly promising strategies.
Keywords: Cerenkov radiation; X-ray; bioluminescence; chemiluminescence; photodynamic therapy; photonanomedicines; proton therapy; sonodynamic therapy; tumor; two-photon; ultrasound therapy; upconversion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bhandari C., Guirguis M., Savan N.A., Shrivastava N., Oliveira S., Hasan T., Obaid G. What NIR Photodynamic Activation Offers Molecular Targeted Nanomedicines: Perspectives into the Conundrum of Tumor Specificity and Selectivity. Nano Today. 2021;36:101052. doi: 10.1016/j.nantod.2020.101052. - DOI - PMC - PubMed
-
- Hu J.J., Lei Q., Zhang X.Z. Recent Advances in Photonanomedicines for Enhanced Cancer Photodynamic Therapy. Prog. Mater. Sci. 2020;114:100685. doi: 10.1016/j.pmatsci.2020.100685. - DOI
-
- Obaid G., Jin W., Bano S., Kessel D., Hasan T. Nanolipid Formulations of Benzoporphyrin Derivative: Exploring the Dependence of Nanoconstruct Photophysics and Photochemistry on Their Therapeutic Index in Ovarian Cancer Cells. Photochem. Photobiol. 2019;95:364–377. doi: 10.1111/php.13002. - DOI - PMC - PubMed
-
- Spring B.Q., Sears R.B., Zheng L.Z., Mai Z., Watanabe R., Sherwood M.E., Schoenfeld D.A., Pogue B.W., Pereira S.P., Villa E. A Photoactivable Multi-Inhibitor Nanoliposome for Tumour Control and Simultaneous Inhibition of Treatment Escape Pathways. Nat. Nanotechnol. 2016;11:378–387. doi: 10.1038/nnano.2015.311. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

