Azacitidine Plus Venetoclax for the Treatment of Relapsed and Newly Diagnosed Acute Myeloid Leukemia Patients
- PMID: 35454930
- PMCID: PMC9028084
- DOI: 10.3390/cancers14082025
Azacitidine Plus Venetoclax for the Treatment of Relapsed and Newly Diagnosed Acute Myeloid Leukemia Patients
Abstract
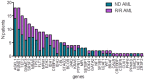
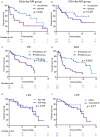
Venetoclax (VEN) belongs the BH3-mimetic class that selectively targets BCL-2, activating apoptosis. The combination of VEN and azacitidine (AZA) has changed the paradigm of treatment of newly diagnosed (ND) acute myeloid leukemia (AML) patients ineligible for intensive chemotherapy. There is scarce evidence for the use of VEN-AZA for relapsed or refractory (R/R) AML. We compared the outcome of 39 R/R AML and 38 ND AML patients treated between 01/20 and 12/21. The median age was 69 (22-86) and 73 (61-81) in the R/R and ND groups, respectively. Adverse cytogenetics were found in 36% of patients in the R/R group and 59% of patients in the ND group. Overall response rate was 37% in R/R AML, including 13% CR, 8% CRi, 3% PR and 13% MLFS, and 58% in the ND AML, including 32% CR, 13% CRi and 13% MLFS. Adverse cytogenetics was associated with treatment failure in the R/R group (Relative Risk = 0.13, p = 0.005). Median overall survival (OS) was 5.9 months in the R/R group and 9.4 months in the ND group. Median OS was 2.2 months in the adverse cytogenetics group versus 8.7 months in the intermediate cytogenetics group in the R/R group (p = 0.02). Median leukemia-free survival was not different between the two groups (9.4 months and 10.3 months), indicating that VEN-AZA can be an efficient salvage treatment for selected R/R AML patients. In conclusion, VEN-AZA is a promising treatment for ND AML and for selected R/R AML patients.
Keywords: acute myeloid leukemia; venetoclax.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. - DOI - PubMed
-
- Pan R., Hogdal L.J., Benito J.M., Bucci D., Han L., Borthakur G., Cortes J., De Angelo D.J., De Bose L., Mu H., et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. - DOI - PMC - PubMed
-
- DiNardo C.D., Pratz K.W., Letai A., Jonas B., Wei A.H., Thirman M., Arellano M., Frattini M.G., Kantarjian H., Popovic R., et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. doi: 10.1016/S1470-2045(18)30010-X. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

