Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects
- PMID: 35455245
- PMCID: PMC9027673
- DOI: 10.3390/vaccines10040497
Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects
Abstract
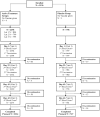
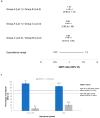
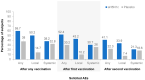
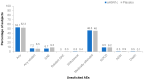
A cell-based process may be better suited for vaccine production during a highly pathogenic avian influenza (HPAI) pandemic. This was a phase 3, randomized, controlled, observer-blind, multicenter study evaluated safety, immunogenicity, and lot-to-lot consistency of two doses of a MF59-adjuvanted, H5N1 influenza pandemic vaccine manufactured on a cell culture platform (aH5N1c) in 3196 healthy adult subjects, stratified into two age groups: 18 to <65 and ≥65 years. Immunogenicity was measured using hemagglutination inhibition (HI) titers. HI antibody responses increased after the first aH5N1c vaccine dose, and 3 weeks after the second vaccination (Day 43), age-appropriate US Center for Biologics Evaluation and Research (CBER) and former European Medicines Authority Committee for Medicinal Products for Human Use (EMA CHMP) immunogenicity criteria were met. Six months after the first vaccination, HI titers were above baseline but no longer met CBER and CHMP criteria. No relevant changes over time were seen in placebo subjects. Solicited AEs were more frequent in the active treatment than the placebo group, primarily due to injection site pain. No serious adverse events (SAEs) related to aH5N1c- were reported. aH5N1c influenza vaccine elicited high levels of antibodies following two vaccinations administered 21 days apart and met both CBER and former CHMP immunogenicity criteria at Day 43 among both younger and older adults with a clinically acceptable safety profile. Consistency of the three consecutive aH5N1c vaccine lots was demonstrated (NCT02839330).
Keywords: H5N1; adult; cell culture-derived; elderly; influenza; pandemic; vaccine.
Conflict of interest statement
J.P. received study fees paid to his Institution by Seqirus, Inc.; E.V., M.H., are employees of Seqirus, USA; E.V.T. is an employee of Seqirus, The Netherlands.
Figures
Similar articles
-
Safety and Immunogenicity of MF59-Adjuvanted Cell Culture-Derived A/H5N1 Subunit Influenza Virus Vaccine: Dose-Finding Clinical Trials in Adults and the Elderly.Open Forum Infect Dis. 2019 Mar 1;6(4):ofz107. doi: 10.1093/ofid/ofz107. eCollection 2019 Apr. Open Forum Infect Dis. 2019. PMID: 30968056 Free PMC article.
-
Safety, Tolerability and Immunogenicity of an MF59-adjuvanted, Cell Culture-derived, A/H5N1, Subunit Influenza Virus Vaccine: Results From a Dose-finding Clinical Trial in Healthy Pediatric Subjects.Pediatr Infect Dis J. 2019 Jul;38(7):757-764. doi: 10.1097/INF.0000000000002345. Pediatr Infect Dis J. 2019. PMID: 31194712 Clinical Trial.
-
Antibody responses against heterologous A/H5N1 strains for an MF59-adjuvanted cell culture-derived A/H5N1 (aH5N1c) influenza vaccine in healthy pediatric subjects.Vaccine. 2021 Nov 16;39(47):6930-6935. doi: 10.1016/j.vaccine.2021.10.010. Epub 2021 Oct 25. Vaccine. 2021. PMID: 34711436 Clinical Trial.
-
Antibody responses against heterologous H5N1 strains for an MF59-adjuvanted cell culture-derived H5N1 (aH5n1c) influenza vaccine in adults and older adults.Hum Vaccin Immunother. 2023 Dec 31;19(1):2193119. doi: 10.1080/21645515.2023.2193119. Epub 2023 Apr 14. Hum Vaccin Immunother. 2023. PMID: 37057755 Free PMC article.
-
Analyses of Safety Profile and Homologous Antibody Responses to a Mammalian Cell-Based, MF59-Adjuvanted, A/H5N1, Pandemic Influenza Vaccine across Four Phase II/III Clinical Trials in Healthy Children, Adults, and Older Adults.Vaccines (Basel). 2021 Dec 11;9(12):1468. doi: 10.3390/vaccines9121468. Vaccines (Basel). 2021. PMID: 34960214 Free PMC article.
Cited by
-
Pandemic preparedness through vaccine development for avian influenza viruses.Hum Vaccin Immunother. 2024 Dec 31;20(1):2347019. doi: 10.1080/21645515.2024.2347019. Epub 2024 May 28. Hum Vaccin Immunother. 2024. PMID: 38807261 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention 2009 H1N1 Pandemic (H1N1pdm09 Virus) [(accessed on 12 April 2021)]; Available online: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html.
-
- Webby R.J., Webster R.G. Are we ready for Pandemic Influenza? In: Knobler S., Mahmoud A., Lemon S., Mack A., Sivitz L.K.O., editors. Learning from SARS: Preparing for the Next Disease Outbreak Workshop Summary. The National Academies Press; Washington, DC, USA: 2004. - PubMed
-
- World Health Organization Human Infection with Avian Influenza A(H5) Viruses. Avian Influenza Weekly Update Number 784. [(accessed on 12 April 2021)]. Available online: https://www.who.int/docs/default-source/wpro---documents/emergency/surve....
-
- Homeland Security Council . National Strategy for Pandemic Influenza. Department of Homeland Security; Washington, DC, USA: 2005.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

