Humoral Response in Hemodialysis Patients Following COVID-19 Vaccination and Breakthrough Infections during Delta and Omicron Variant Predominance
- PMID: 35455248
- PMCID: PMC9028840
- DOI: 10.3390/vaccines10040498
Humoral Response in Hemodialysis Patients Following COVID-19 Vaccination and Breakthrough Infections during Delta and Omicron Variant Predominance
Abstract
Background: The advancement of COVID-19 vaccination programs globally has been viewed as an integral strategy to reduce both the number of COVID-19 cases and consequential complications of COVID-19, particularly for high-risk patient groups. There are limited data on the antibody response and protection from disease infection and severity in patients requiring hemodialysis (HD) following COVID-19 vaccination during the Delta and Omicron variant predominance. We conducted a study aiming to evaluate humoral immunity derived from two different COVID-19 vaccines administered to our in-centre HD population and investigated the characteristics of breakthrough COVID-19 infections occurring post-vaccination within this population.
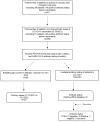
Methods: This is a prospective observational study including patients receiving HD at Salford Royal Hospital. The first and second doses of COVID-19 vaccinations (Pfizer BioNTech BNT162b2 or Oxford AstraZeneca ChAdOx1 nCoV-19) were administered to this patient cohort since January 2021. The incidence of any breakthrough COVID-19 infections occurring in double vaccinated patients between 1 April 2021 and 15 January 2022 was recorded. Patients were screened weekly with nasal and pharyngeal nasopharyngeal swabs for real-time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) for COVID-19, whilst SARS-CoV-2 antibody testing was performed alongside monthly routine HD bloods.
Results: Four hundred eleven patients receiving HD were included in this study, of which 170 of 178 patients (95.5%) with available data on antibody status following two doses of the Pfizer BioNTech BNT162b2 vaccination had detectable antibody response, whilst this was the case for 97 of 101 patients (96.1%) who received two doses of the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine. For 12 seronegative patients who received a booster vaccine (third dose), nine seroconverted, while one remained negative and two were not tested. No statistically significant differences were observed with regards to antibody status between those receiving Pfizer BioNTech BNT162b2 and Oxford AstraZeneca ChAdOx1 nCoV-19 vaccines. Sixty-three of 353 patients with two doses of COVID-19 vaccination had breakthrough COVID-19 infection (40 during Delta and 23 during Omicron variant predominance). Of the 40 patients during the delta period, five were admitted into hospital and there were two reported deaths due to COVID-19-related illness. There were no COVID-19 associated hospitalizations or deaths during the Omicron variant predominance.
Conclusions: The vast majority of HD patients who received two doses of the Pfizer BioNTech BNT162b2 or Oxford AstraZeneca ChAdOx1 nCoV-19 vaccinations developed detectable antibody responses. Our results support the value of booster vaccination with mRNA-based COVID-19 vaccine in HD patients and highlight the need for ongoing surveillance programmes with rRT-PCR and antibody testing for timely detection of positive cases.
Keywords: COVID-19 vaccination; Delta variant; Omicron variant; breakthrough infections; hemodialysis; humoral response.
Conflict of interest statement
The authors declare no conflict of interest for this work.
Figures


Similar articles
-
Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant.N Engl J Med. 2022 Apr 21;386(16):1532-1546. doi: 10.1056/NEJMoa2119451. Epub 2022 Mar 2. N Engl J Med. 2022. PMID: 35249272 Free PMC article.
-
[COVID-19 infections and effectiveness of the vaccination among healthcare workers].Orv Hetil. 2023 Feb 5;164(5):163-171. doi: 10.1556/650.2023.32709. Print 2023 Feb 5. Orv Hetil. 2023. PMID: 36739547 Hungarian.
-
Effectiveness of the BNT162b2 (Pfizer-BioNTech) and the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccines for reducing susceptibility to infection with the Delta variant (B.1.617.2) of SARS-CoV-2.BMC Infect Dis. 2022 Mar 20;22(1):270. doi: 10.1186/s12879-022-07239-z. BMC Infect Dis. 2022. PMID: 35307024 Free PMC article.
-
The Omicron variant wave: Where are we now and what are the prospects?J Chin Med Assoc. 2023 Feb 1;86(2):135-137. doi: 10.1097/JCMA.0000000000000863. Epub 2022 Dec 13. J Chin Med Assoc. 2023. PMID: 36524941 Review.
-
Implication of the emergence of the delta (B.1.617.2) variants on vaccine effectiveness.Infection. 2022 Jun;50(3):583-596. doi: 10.1007/s15010-022-01759-1. Epub 2022 Feb 3. Infection. 2022. PMID: 35113351 Free PMC article. Review.
Cited by
-
Immunogenicity and Safety of the Three-Dose COVID-19 Vaccine Regimen in Patients Receiving Renal Replacement Therapy: A Systematic Review and Meta-Analysis.Kidney Dis (Basel). 2024 Jan 19;10(2):107-117. doi: 10.1159/000536308. eCollection 2024 Apr. Kidney Dis (Basel). 2024. PMID: 38751793 Free PMC article. Review.
-
Correlation of Breakthrough Infection During the Omicron Wave With Seropositivity of Vaccinated Patients Undergoing Hemodialysis.Cureus. 2022 Sep 18;14(9):e29296. doi: 10.7759/cureus.29296. eCollection 2022 Sep. Cureus. 2022. PMID: 36277581 Free PMC article.
-
Effect of Third and Fourth mRNA-Based Booster Vaccinations on SARS-CoV-2 Neutralizing Antibody Titer Formation, Risk Factors for Non-Response, and Outcome after SARS-CoV-2 Omicron Breakthrough Infections in Patients on Chronic Hemodialysis: A Prospective Multicenter Cohort Study.J Clin Med. 2022 Jun 2;11(11):3187. doi: 10.3390/jcm11113187. J Clin Med. 2022. PMID: 35683580 Free PMC article.
-
Humoral and cellular immunity against different SARS-CoV-2 variants in patients with chronic kidney disease.Sci Rep. 2023 Nov 15;13(1):19932. doi: 10.1038/s41598-023-47130-8. Sci Rep. 2023. PMID: 37968273 Free PMC article.
-
Two versus three doses of COVID-19 vaccine and post-vaccination COVID-19 infection in hemodialysis patients.Infect Prev Pract. 2024 Jan 10;6(1):100338. doi: 10.1016/j.infpip.2024.100338. eCollection 2024 Mar. Infect Prev Pract. 2024. PMID: 38304200 Free PMC article.
References
-
- UK Kidney Association UK Kidney Association Guidance on COVID-19 Vaccination in Highly Vulnerable People with Kidney Disease. 2021. [(accessed on 10 February 2022)]. Available online: https://ukkidney.org/sites/renal.org/files/UKKA%20COVID19%20Vaccination%....
-
- Speer C., Göth D., Benning L., Buylaert M., Schaier M., Grenz J., Nusshag C., Kälble F., Kreysing M., Reichel P., et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021;16:1073–1082. doi: 10.2215/CJN.03700321. - DOI - PMC - PubMed
-
- Yau K., Abe K.T., Naimark D., Oliver M.J., Perl J., Leis J.A., Bolotin S., Tran V., Mullin S.I., Shadowitz E., et al. Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis. JAMA Netw. Open. 2021;4:e2123622. doi: 10.1001/jamanetworkopen.2021.23622. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

