Effectiveness, Adverse Events, and Immune Response Following Double Vaccination with BNT162b2 in Staff at the National Comprehensive Cancer Center (NCCC)
- PMID: 35455308
- PMCID: PMC9026370
- DOI: 10.3390/vaccines10040558
Effectiveness, Adverse Events, and Immune Response Following Double Vaccination with BNT162b2 in Staff at the National Comprehensive Cancer Center (NCCC)
Abstract
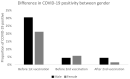
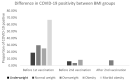
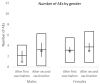
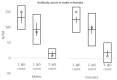
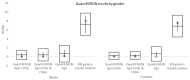
Vaccination remains the leading strategy against COVID-19 worldwide. BNT162b2 is among the first licensed vaccines with high effectiveness. However, the role of antibody and cell immunity response monitoring after vaccination remains unclear. We conducted a 6-month prospective study involving the employees of NCCC in Slovakia, who were tested for IgG antibody and cell immune responses after double vaccination with BNT162b2. IgG antibodies were detected at 3, 7, and 26 weeks, respectively. At 6 months, blood samples were tested by two different interferon-γ release assays to determine responses to spike protein antigen and nucleocapsid protein antigen of the novel coronavirus. Results were stratified by gender and body mass index (BMI). Statistical significance was set at p = 0.05. The medical records of 94 respondents (71 females) were analyzed. The mean age was 40.2 years and the mean BMI was 26.4 kg/m2. At 6 months after double vaccination, effectiveness was 97.9%. The side effects of the BNT162b2 vaccine were similar after both doses, with no serious adverse events or new safety signals recorded. The IgG index declined rapidly (p < 0.0001), and 42.6% of subjects had positive and 57.4% borderline or negative immune cell response at 6 months (p < 0.0001). Both T cell activation and IgG counts were lower in morbidly obese patients when compared to some other BMI categories. This study confirmed an acceptable toxicity profile and the high efficacy of BNT162b2 despite a rapid decline of IgG level and negative cell-mediated immunity response in most subjects. An individualized approach to vaccination could be considered in morbidly obese individuals.
Keywords: BNT162b2; IgG antibodies; adverse events; cell-mediated immunity; effectiveness.
Conflict of interest statement
The authors declare no conflict of interests related to this study.
Figures



References
-
- World Health Organization WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. [(accessed on 13 February 2022)]. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at....
-
- World Health Organization . Advice on the Use of Masks in the Context of COVID-19: Interim Guidance. World Health Organization; Geneva, Switzerland: 2020. No. WHO/2019-nCoV/IPC_Masks/2020.4.
LinkOut - more resources
Full Text Sources

