The Fluctuation Trend of Serum Anti-SARS-CoV-2 IgM/IgG Antibodies Seroprevalence in the Non-COVID-19 Infected Population and Correlation with Peripheral Blood Leukocyte Parameters in Beijing, China, 2021: A Real-World Study
- PMID: 35455320
- PMCID: PMC9032992
- DOI: 10.3390/vaccines10040571
The Fluctuation Trend of Serum Anti-SARS-CoV-2 IgM/IgG Antibodies Seroprevalence in the Non-COVID-19 Infected Population and Correlation with Peripheral Blood Leukocyte Parameters in Beijing, China, 2021: A Real-World Study
Abstract
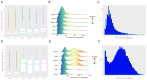
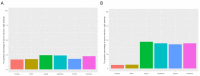
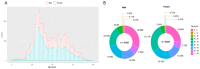
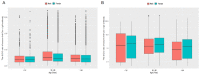
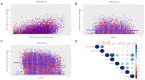
Since 2019, the coronavirus disease 2019 (COVID-19) global pandemic has caused more than 300 million cases of disease and 5 million deaths. Vaccination has been widely accepted as the most effective measure for the prevention and control of this disease. However, there is little understanding about serum anti-SARS-CoV-2 IgM/IgG levels after inactivated vaccination as well as the relationship with peripheral blood leukocytes in the non-COVID-19 infected population. A total of 16,335 male and 22,302 female participants were recruited in this study, which was conducted in the Peking University Third Hospital located in Beijing (China). The level and seroprevalence of serum anti-SARS-CoV-2 receptor-binding domain (RBD) IgM/IgG and the association with peripheral blood leukocytes classification were investigated. With an increase in the number and percentage of full immunization of COVID-19 vaccinations in Beijing, serum anti-SARS-CoV-2 IgG antibodies levels and seroprevalence were significantly elevated (p < 0.01). The serum anti-SARS-CoV-2 IgG antibodies of 60 years and older persons were significantly lower than that of individuals that are 18~60 years old (p < 0.01), and there was a positive relationship between serum anti-SARS-CoV-2 IgG antibodies levels and peripheral blood lymphocyte count. The investigation of serum anti-SARS-CoV-2 IgM/IgG antibodies and the peripheral hematological index may prompt and help understand the adaptive immune response of vaccination.
Keywords: COVID-19; IgM and IgG antibody; SARS-CoV-2; vaccine.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Harvey R.A., Rassen J.A., Kabelac C.A., Turenne W., Leonard S., Klesh R., Meyer W.A., Kaufman H.W., Anderson S., Cohen V.I., et al. Real-World Data Suggest Antibody Positivity to SARS-CoV-2 Is Associated with a Decreased Risk of Future Infection. medRxiv. 2020 doi: 10.1101/2020.12.18.20248336. - DOI
-
- He Z., Ren L., Yang J., Guo L., Feng L., Ma C., Wang X., Leng Z., Tong X., Zhou W., et al. Seroprevalence and Humoral Immune Durability of Anti-SARS-CoV-2 Antibodies in Wuhan, China: A Longitudinal, Population-Level, Cross-Sectional Study. Lancet. 2021;397:1075–1084. doi: 10.1016/S0140-6736(21)00238-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

