Local Response and Barrier Recovery in Elderly Skin Following the Application of High-Density Microarray Patches
- PMID: 35455332
- PMCID: PMC9031416
- DOI: 10.3390/vaccines10040583
Local Response and Barrier Recovery in Elderly Skin Following the Application of High-Density Microarray Patches
Abstract
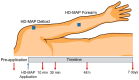
The high-density microneedle array patch (HD-MAP) is a promising alternative vaccine delivery system device with broad application in disease, including SARS-CoV-2. Skin reactivity to HD-MAP applications has been extensively studied in young individuals, but not in the >65 years population, a risk group often requiring higher dose vaccines to produce protective immune responses. The primary aims of the present study were to characterise local inflammatory responses and barrier recovery to HD-MAPs in elderly skin. In twelve volunteers aged 69−84 years, HD-MAPs were applied to the forearm and deltoid regions. Measurements of transepidermal water loss (TEWL), dielectric permittivity and erythema were performed before and after HD-MAP application at t = 10 min, 30 min, 48 h, and 7 days. At all sites, TEWL (barrier damage), dielectric permittivity (superficial water);, and erythema measurements rapidly increased after HD-MAP application. After 7 days, the mean measures had recovered toward pre-application values. The fact that the degree and chronology of skin reactivity and recovery after HD-MAP was similar in elderly skin to that previously reported in younger adults suggests that the reactivity basis for physical immune enhancement observed in younger adults will also be achievable in the older population.
Keywords: dielectric permittivity; evaporimetry; microneedles; polarisation spectroscopy; skin barrier integrity; skin reactivity; vaccination.
Conflict of interest statement
B.B. is a Vaxxas employee. D.A.M. and C.D.A. have undertaken consultant work for Vaxxas. J.H. has a royalty agreement with the company (WheelsBridge AB) making the TiVi equipment. C.D.A. is a shareholder in WheelsBridge AB. The remaining authors declare no conflict. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Hesse E.M., Atanasoff S., Hibbs B.F., Adegoke O.J., Ng C., Marquez P., Osborn M., Su J.R., Moro P.L., Shimabukuro T., et al. Shoulder Injury Related to Vaccine Administration (SIRVA): Petitioner claims to the National Vaccine Injury Compensation Program, 2010–2016. Vaccine. 2020;38:1076–1083. doi: 10.1016/j.vaccine.2019.11.032. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

