Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response
- PMID: 35455334
- PMCID: PMC9029590
- DOI: 10.3390/vaccines10040585
Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response
Abstract
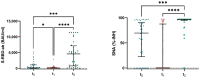
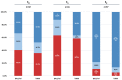
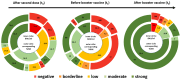
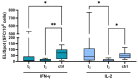
The long-term effect of protection by two doses of SARS-CoV-2 vaccination in patients receiving chronic intermittent hemodialysis (CIHD) is an urging question. We investigated the humoral and cellular immune response of 42 CIHD patients who had received two doses of SARS-CoV-2 vaccine, and again after a booster vaccine with mRNA-1273 six months later. We measured antibody levels and SARS-CoV-2-specific surrogate neutralizing antibodies (SNA). Functional T cell immune response to vaccination was assessed by quantifying interferon-γ (IFN-γ) and IL-2 secreting T cells specific for SARS-CoV-2 using an ELISpot assay. Our data reveal a moderate immune response after the second dose of vaccination, with significantly decreasing SARS-CoV-2-specific antibody levels and less than half of the study group showed neutralizing antibodies six months afterwards. Booster vaccines increased the humoral response dramatically and led to a response rate of 89.2% for antibody levels and a response rate of 94.6% for SNA. Measurement in a no response/low response (NR/LR) subgroup of our cohort, which differed from the whole group in age and rate of immunosuppressive drugs, indicated failure of a corresponding T cell response after the booster vaccine. We strongly argue in favor of a regular testing of surrogate neutralizing antibodies and consecutive booster vaccinations for CIHD patients to provide a stronger and persistent immunity.
Keywords: COVID-19; SARS-CoV-2; T cell response; booster; hemodialysis; mRNA-1273; seroconversion; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effect of Third and Fourth mRNA-Based Booster Vaccinations on SARS-CoV-2 Neutralizing Antibody Titer Formation, Risk Factors for Non-Response, and Outcome after SARS-CoV-2 Omicron Breakthrough Infections in Patients on Chronic Hemodialysis: A Prospective Multicenter Cohort Study.J Clin Med. 2022 Jun 2;11(11):3187. doi: 10.3390/jcm11113187. J Clin Med. 2022. PMID: 35683580 Free PMC article.
-
Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study.Vaccines (Basel). 2023 Jan 25;11(2):260. doi: 10.3390/vaccines11020260. Vaccines (Basel). 2023. PMID: 36851137 Free PMC article.
-
Evolution of SARS-CoV-2-Neutralizing Antibodies after Two Standard Dose Vaccinations, Risk Factors for Non-Response and Effect of a Third Dose Booster Vaccination in Non-Responders on Hemodialysis: A Prospective Multi-Centre Cohort Study.J Clin Med. 2021 Oct 30;10(21):5113. doi: 10.3390/jcm10215113. J Clin Med. 2021. PMID: 34768631 Free PMC article.
-
Immunogenicity of COVID-19 Tozinameran Vaccination in Patients on Chronic Dialysis.Front Immunol. 2021 Jun 30;12:690698. doi: 10.3389/fimmu.2021.690698. eCollection 2021. Front Immunol. 2021. PMID: 34276681 Free PMC article.
-
SARS-CoV-2-mRNA Booster Vaccination Reverses Non-Responsiveness and Early Antibody Waning in Immunocompromised Patients - A Phase Four Study Comparing Immune Responses in Patients With Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease.Front Immunol. 2022 May 12;13:889138. doi: 10.3389/fimmu.2022.889138. eCollection 2022. Front Immunol. 2022. PMID: 35634285 Free PMC article. Clinical Trial.
Cited by
-
Longitudinal cellular and humoral immune responses after triple BNT162b2 and fourth full-dose mRNA-1273 vaccination in haemodialysis patients.Front Immunol. 2022 Oct 6;13:1004045. doi: 10.3389/fimmu.2022.1004045. eCollection 2022. Front Immunol. 2022. PMID: 36275672 Free PMC article.
-
Immunogenicity of mRNA-1273 and BNT162b2 in Immunocompromised Patients: Systematic Review and Meta-analysis Using GRADE.Infect Dis Ther. 2024 Jul;13(7):1419-1438. doi: 10.1007/s40121-024-00987-2. Epub 2024 May 27. Infect Dis Ther. 2024. PMID: 38802704 Free PMC article. Review.
-
Effect of Third and Fourth mRNA-Based Booster Vaccinations on SARS-CoV-2 Neutralizing Antibody Titer Formation, Risk Factors for Non-Response, and Outcome after SARS-CoV-2 Omicron Breakthrough Infections in Patients on Chronic Hemodialysis: A Prospective Multicenter Cohort Study.J Clin Med. 2022 Jun 2;11(11):3187. doi: 10.3390/jcm11113187. J Clin Med. 2022. PMID: 35683580 Free PMC article.
-
Increasing but insufficient neutralizing activity against Omicron-BA.1 after a second booster dose of mRNA-1273 vaccine in chronic haemodialysis patients.Clin Kidney J. 2022 Sep 16;15(12):2346-2348. doi: 10.1093/ckj/sfac211. eCollection 2022 Dec. Clin Kidney J. 2022. PMID: 36381372 Free PMC article. No abstract available.
-
COVID-19 Vaccination Among Patients Receiving Maintenance Renal Replacement Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future.J Infect Dis. 2023 Aug 4;228(Suppl 1):S46-S54. doi: 10.1093/infdis/jiad162. J Infect Dis. 2023. PMID: 37539761 Free PMC article.
References
-
- Losappio V., Franzin R., Infante B., Godeas G., Gesualdo L., Fersini A., Castellano G., Stallone G. Molecular Mechanisms of Premature Aging in Hemodialysis: The Complex Interplay Between Innate and Adaptive Immune Dysfunction. Int. J. Mol. Sci. 2020;21:3422. doi: 10.3390/ijms21103422. - DOI - PMC - PubMed
-
- Hilbrands L.B., Duivenvoorden R., Vart P., Franssen C.F., Hemmelder M.H., Jager K.J., Kieneker L.M., Noordzij M., Pena M.J., Gansevoort R.T., et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

