Safety of Global SARS-CoV-2 Vaccines, a Meta-Analysis
- PMID: 35455344
- PMCID: PMC9030038
- DOI: 10.3390/vaccines10040596
Safety of Global SARS-CoV-2 Vaccines, a Meta-Analysis
Abstract
(1) Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines were developed in only a short amount of time and were widely distributed. We conducted this meta-analysis to understand the safety of SARS-CoV-2 vaccines. (2) Methods: We searched the corresponding literature published from 1 January 2020 to 20 October 2021. Information of adverse events (AEs) of each selected work was collected. The quality and bias of studies was evaluated, and meta-analysis was carried out by using Stata 17.0. (3) Results: Totally, 11,451 articles were retrieved, and 53 of them were included for analysis. The incidence rate of AEs was 20.05-94.48%. The incidence rate of vascular events increased after viral vector vaccination, while the incidence rate of vascular events decreased after mRNA vaccination. Viral vector vaccine had a higher AE rate compared to mRNA vaccines and inactivated vaccines. In most circumstances, the incidence of AEs was higher in older people, female and after the second dose. The sensitivity of meta-analysis was acceptable; however, the literature was subject to a certain publication bias. (4) Conclusions: The safety of SARS-CoV-2 vaccines was acceptable. The incidence of allergic symptoms and cardiovascular and cerebrovascular symptoms was low. Viral vector vaccine had a higher risk of leading to thrombosis events. The understanding of SARS-CoV-2 vaccine AEs should be enhanced, so as to promote the vaccination.
Keywords: COVID-19; adverse events; meta-analysis; safety; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
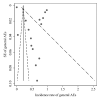
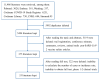


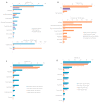
References
-
- WHO WHO Coronavirus (COVID-19) Dashboard. [(accessed on 11 March 2022)]. Available online: https://covid19.who.int/
-
- WHO Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Statement on the Second Meeting of the International Health Regulations. 2005. [(accessed on 27 November 2021)]. Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting...
-
- UNICEF COVID-19 Vaccine Market Dashboard. [(accessed on 30 November 2021)]. Available online: https://www.unicef.org/supply/covid-19-vaccine-market-dashboard.
-
- Huan Y., Bi Y. Research progress and prospect of vaccines for the coronavirus disease 2019 (COVID-19) Sci. Sin. Vitae. 2022;52:237–248. doi: 10.1360/SSV-2021-0082. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

