Mycobacterium bovis Wild-Type BCG or Recombinant BCG Secreting Murine IL-18 (rBCG/IL-18) Strains in Driving Immune Responses in Immunocompetent or Immunosuppressed Mice
- PMID: 35455364
- PMCID: PMC9030902
- DOI: 10.3390/vaccines10040615
Mycobacterium bovis Wild-Type BCG or Recombinant BCG Secreting Murine IL-18 (rBCG/IL-18) Strains in Driving Immune Responses in Immunocompetent or Immunosuppressed Mice
Abstract
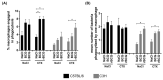
Mycobacterium tuberculosis infections remain a global health problem in immunosuppressed patients. The effectiveness of BCG (Bacillus Calmette−Guérin), an anti-tuberculosis vaccine, is unsatisfactory. Finding a new vaccine candidate is a priority. We compared numerous immune markers in BCG-susceptible C57BL/6 and BCG-resistant C3H mice who had been injected with 0.9% NaCl (control) or with wild-type BCG or recombinant BCG secreting interleukin (IL)-18 (rBCG/IL-18) and in immunized mice who were immunocompromised with cyclophosphamide (CTX). The inoculation of rBCG/IL-18 in immunocompetent mice increased the percentage of bone marrow myeloblasts and promyelocytes, which were further elevated in the rBCG/IL-18/CTX-treated mice: C57BL/6 mice—3.0% and 11.4% (control) vs. 18.6% and 42.4%, respectively; C3H mice—1.1% and 7.7% (control) vs. 18.4% and 44.9%, respectively, p < 0.05. The bone marrow cells showed an increased mean fluorescence index (MFI) in the CD34 adhesion molecules: C57BL/6 mice—4.0 × 103 (control) vs. 6.2 × 103; C3H mice—4.0 × 103 (control) vs. 8.0 × 103, p < 0.05. Even in the CTX-treated mice, the rBCG/IL-18 mobilized macrophages for phagocytosis, C57BL/6 mice—4% (control) vs. 8%; C3H mice—2% (control) vs. 6%, and in immunocompetent mice, C57BL/6 induced the spleen homing of effector memory CD4+ and CD8+ T cells (TEM), 15% (control) vs. 28% and 8% (control) vs. 22%, respectively, p < 0.05. In conclusion, rBCG/IL-18 effectively induced selected immune determinants that were maintained even in immunocompromised mice.
Keywords: BCG; C3H; C57BL/6; IL-18; cyclophosphamide; immunity; immunosuppression.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
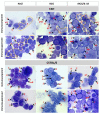
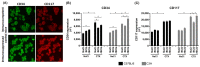

References
-
- Global Tuberculosis Report WHO 2021. [(accessed on 17 February 2022)]. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

