Assessment of Seroconversion after SARS-CoV-2 Vaccination in Patients with Lung Cancer
- PMID: 35455367
- PMCID: PMC9031406
- DOI: 10.3390/vaccines10040618
Assessment of Seroconversion after SARS-CoV-2 Vaccination in Patients with Lung Cancer
Abstract
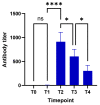
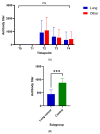
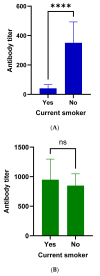
Background: SARS-CoV-2 mortality rates are significantly higher in patients with lung cancer compared with the general population. However, little is known on their immunization status after vaccination. Methods: To evaluate the humoral response (seroconversion) of patients with lung cancer following vaccination against SARS-COV-2 (Group A), we obtained antibodies against SARS-CoV-2 spike (S) protein both at baseline and at different time points after the first dose of SARS-CoV-2 vaccine (two to three weeks [T1], six weeks ± one week [T2], 12 weeks ± three weeks [T3], and 24 weeks ± three weeks [T4]). Antibodies were also acquired from a control cohort of non-lung cancer patients (Group B) as well as a third cohort containing healthy controls (Group C) at all time points and at T4, respectively, to make comparisons with Group A. Analysis of antibody response at different time points, association with clinicopathologic parameters, and comparisons with control groups were performed. Results: A total of 125 patients with lung cancer were included in the analysis (96 males [74.3%], median age of 68 years [46−91]. All study participants received two vaccine doses (BNT162b2, mRNA-1273, AZD1222). Analysis of anti-SARS-CoV-2 S antibody titers showed minimal response at T1 (0.4 [0.4−48.6] IU/mL). Antibody response peaked at T2 (527.0 [0.4−2500] IU/mL) and declined over T3 (323.0 [0.4−2500] IU/mL) and T4 (141.0 [0.4−2500] IU/mL). Active smokers had lower antibody titers at T2 (p = 0.04), T3 (p = 0.04), and T4 (p < 0.0001) compared with former or never smokers. Peak antibody titers were not associated with any other clinicopathologic characteristic. No significant differences were observed compared with Group B. However, lung cancer patients exhibited significantly decreased antibody titers compared with Group C at T4 (p < 0.0001). Conclusions: Lung cancer patients demonstrate sufficient antibody response six weeks after the first dose of vaccine against SARS-CoV-2 when vaccinated with two-dose regimens. Rapidly declining antibody titers six weeks after the first dose underline the need for a third dose three months later, in patients with lung cancer, and especially active smokers.
Keywords: COVID-19; SARS-CoV-2; antibodies; humoral immunity; lung cancer; seroconversion; vaccination.
Conflict of interest statement
The authors declare no conflict of interest for this study. Roche diagnostics had no role in the design, execution, interpretation or writing of the study.
Figures
References
-
- Word Health Organization Who Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. [(accessed on 5 January 2022)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., Yee N.T.S., Liu C., Nerurkar S.N., Kai J.C.Y., et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

