Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner
- PMID: 35455369
- PMCID: PMC9030172
- DOI: 10.3390/vaccines10040620
Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner
Abstract
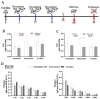
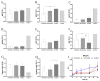
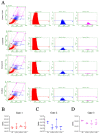
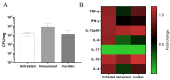
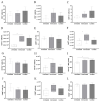
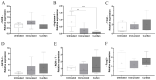
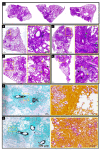
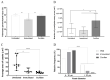
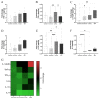
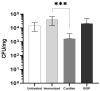
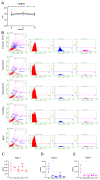
The low efficacy and side effects associated with antifungal agents have highlighted the importance of developing immunotherapeutic approaches to treat Cryptococcus gattii infection. We developed an immunization strategy that uses selective Dectin-1 agonist as an adjuvant. BALB/c or C57BL/6 mice received curdlan or β-glucan peptide (BGP) before immunization with heat-killed C. gattii, and the mice were infected with viable C. gattii on day 14 post immunization and euthanized 14 days after infection. Adjuvant curdlan restored pulmonary tumor necrosis factor- α (TNF-α) levels, as induced by immunization with heat-killed C. gattii. The average area and relative frequency of C. gattii titan cells in the lungs of curdlan-treated BALB/c mice were reduced. However, this did not reduce the pulmonary fungal burden or decrease the i0,nflammatory infiltrate in the pulmonary parenchyma of BALB/c mice. Conversely, adjuvant curdlan induced high levels of interferon-γ (IFN-γ) and interleukin (IL)-10 and decreased the C. gattii burden in the lungs of C57BL/6 mice, which was not replicated in β-glucan peptide-treated mice. The adjuvant curdlan favors the control of C. gattii infection depending on the immune response profile of the mouse strain. This study will have implications for developing new immunotherapeutic approaches to treat C. gattii infection.
Keywords: Cryptococcus gattii; Dectin-1; curdlan; immunotherapy; β-glucan peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
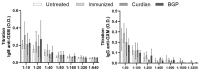
Similar articles
-
Adjuvant Pam3CSk4 does not improve the immunization against Cryptococcus gattii infection in C57BL/6 mice.PeerJ. 2023 Jan 31;11:e14778. doi: 10.7717/peerj.14778. eCollection 2023. PeerJ. 2023. PMID: 36743957 Free PMC article.
-
Dendritic cell-based immunization ameliorates pulmonary infection with highly virulent Cryptococcus gattii.Infect Immun. 2015 Apr;83(4):1577-86. doi: 10.1128/IAI.02827-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644007 Free PMC article.
-
Dendritic Cell-Based Vaccine Against Fungal Infection.Methods Mol Biol. 2016;1403:537-49. doi: 10.1007/978-1-4939-3387-7_30. Methods Mol Biol. 2016. PMID: 27076152
-
Pulmonary Iron Limitation Induced by Exogenous Type I IFN Protects Mice from Cryptococcus gattii Independently of T Cells.mBio. 2019 Jun 18;10(3):e00799-19. doi: 10.1128/mBio.00799-19. mBio. 2019. PMID: 31213551 Free PMC article.
-
[Mechanism of Cryptococcus Meningoencephalitis].Med Mycol J. 2016;57(1):J27-32. doi: 10.3314/mmj.57.J27. Med Mycol J. 2016. PMID: 26936349 Review. Japanese.
Cited by
-
Immune evasion by Cryptococcus gattii in vaccinated mice coinfected with C. neoformans.Front Immunol. 2024 Feb 26;15:1356651. doi: 10.3389/fimmu.2024.1356651. eCollection 2024. Front Immunol. 2024. PMID: 38469300 Free PMC article.
-
Preclinical Models for Cryptococcosis of the CNS and Their Characterization Using In Vivo Imaging Techniques.J Fungi (Basel). 2024 Feb 12;10(2):146. doi: 10.3390/jof10020146. J Fungi (Basel). 2024. PMID: 38392818 Free PMC article. Review.
-
Adjuvant Pam3CSk4 does not improve the immunization against Cryptococcus gattii infection in C57BL/6 mice.PeerJ. 2023 Jan 31;11:e14778. doi: 10.7717/peerj.14778. eCollection 2023. PeerJ. 2023. PMID: 36743957 Free PMC article.
-
Adjuvant ArtinM favored the host immunity against Cryptococcus gattii infection in C57BL/6 mice.Immunotherapy. 2024;16(11):733-748. doi: 10.1080/1750743X.2024.2360384. Epub 2024 Jun 28. Immunotherapy. 2024. PMID: 38940276 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

