Neuronal Hyperexcitability and Free Radical Toxicity in Amyotrophic Lateral Sclerosis: Established and Future Targets
- PMID: 35455429
- PMCID: PMC9025031
- DOI: 10.3390/ph15040433
Neuronal Hyperexcitability and Free Radical Toxicity in Amyotrophic Lateral Sclerosis: Established and Future Targets
Abstract
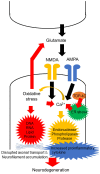
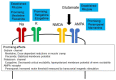
Amyotrophic lateral sclerosis (ALS) is a devastating disease with evidence of degeneration involving upper and lower motor neuron compartments of the nervous system. Presently, two drugs, riluzole and edaravone, have been established as being useful in slowing disease progression in ALS. Riluzole possesses anti-glutamatergic properties, while edaravone eliminates free radicals (FRs). Glutamate is the excitatory neurotransmitter in the brain and spinal cord and binds to several inotropic receptors. Excessive activation of these receptors generates FRs, inducing neurodegeneration via damage to intracellular organelles and upregulation of proinflammatory mediators. FRs bind to intracellular structures, leading to cellular impairment that contributes to neurodegeneration. As such, excitotoxicity and FR toxicities have been considered as key pathophysiological mechanisms that contribute to the cascade of degeneration that envelopes neurons in ALS. Recent advanced technologies, including neurophysiological, imaging, pathological and biochemical techniques, have concurrently identified evidence of increased excitability in ALS. This review focuses on the relationship between FRs and excitotoxicity in motor neuronal degeneration in ALS and introduces concepts linked to increased excitability across both compartments of the human nervous system. Within this cellular framework, future strategies to promote therapeutic development in ALS, from the perspective of neuronal excitability and function, will be critically appraised.
Keywords: amyotrophic lateral sclerosis; cortical excitability; excitotoxicity; free radicals; nerve excitability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Hulisz D. Amyotrophic lateral sclerosis: Disease state overview. Am. J. Manag. Care. 2018;24:S320–S326. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

