Cilia Stimulatory and Antibacterial Activities of T2R Bitter Taste Receptor Agonist Diphenhydramine: Insights into Repurposing Bitter Drugs for Nasal Infections
- PMID: 35455449
- PMCID: PMC9025516
- DOI: 10.3390/ph15040452
Cilia Stimulatory and Antibacterial Activities of T2R Bitter Taste Receptor Agonist Diphenhydramine: Insights into Repurposing Bitter Drugs for Nasal Infections
Abstract
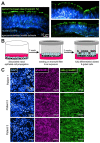
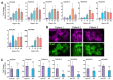
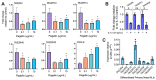
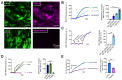
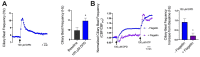
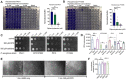
T2R bitter taste receptors in airway motile cilia increase ciliary beat frequency (CBF) and nitric oxide (NO) production. Polymorphisms in some T2Rs are linked to disease outcomes in chronic rhinosinusitis (CRS) and cystic fibrosis (CF). We examined the expression of cilia T2Rs during the differentiation of human nasal epithelial cells grown at air-liquid interface (ALI). The T2R expression increased with differentiation but did not vary between CF and non-CF cultures. Treatment with Pseudomonas aeruginosa flagellin decreased the expression of diphenhydramine-responsive T2R14 and 40, among others. Diphenhydramine increased both NO production, measured by fluorescent dye DAF-FM, and CBF, measured via high-speed imaging. Increases in CBF were disrupted after flagellin treatment. Diphenhydramine impaired the growth of lab and clinical strains of P. aeruginosa, a major pathogen in CF and CF-related CRS. Diphenhydramine impaired biofilm formation of P. aeruginosa, measured via crystal violet staining, as well as the surface attachment of P. aeruginosa to CF airway epithelial cells, measured using colony-forming unit counting. Because the T2R agonist diphenhydramine increases NO production and CBF while also decreasing bacterial growth and biofilm production, diphenhydramine-derived compounds may have potential clinical usefulness in CF-related CRS as a topical therapy. However, utilizing T2R agonists as therapeutics within the context of P. aeruginosa infection may require co-treatment with anti-inflammatories to enhance T2R expression.
Keywords: G protein-coupled receptors; Pseudomonas aeruginosa; Staphylococcus aureus; calcium; chronic rhinosinusitis; ciliary beat frequency; cystic fibrosis; nitric oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Loss of CFTR function is associated with reduced bitter taste receptor-stimulated nitric oxide innate immune responses in nasal epithelial cells and macrophages.Front Immunol. 2023 Jan 18;14:1096242. doi: 10.3389/fimmu.2023.1096242. eCollection 2023. Front Immunol. 2023. PMID: 36742335 Free PMC article.
-
The bitter end: T2R bitter receptor agonists elevate nuclear calcium and induce apoptosis in non-ciliated airway epithelial cells.Cell Calcium. 2022 Jan;101:102499. doi: 10.1016/j.ceca.2021.102499. Epub 2021 Nov 8. Cell Calcium. 2022. PMID: 34839223 Free PMC article.
-
HSP90 Modulates T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages.Cells. 2022 Apr 27;11(9):1478. doi: 10.3390/cells11091478. Cells. 2022. PMID: 35563784 Free PMC article.
-
The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis.Am J Rhinol Allergy. 2013 Jul-Aug;27(4):283-6. doi: 10.2500/ajra.2013.27.3911. Am J Rhinol Allergy. 2013. PMID: 23883809 Review.
-
The pharmacology of bitter taste receptors and their role in human airways.Pharmacol Ther. 2015 Nov;155:11-21. doi: 10.1016/j.pharmthera.2015.08.001. Epub 2015 Aug 10. Pharmacol Ther. 2015. PMID: 26272040 Review.
Cited by
-
Interkingdom Detection of Bacterial Quorum-Sensing Molecules by Mammalian Taste Receptors.Microorganisms. 2023 May 16;11(5):1295. doi: 10.3390/microorganisms11051295. Microorganisms. 2023. PMID: 37317269 Free PMC article. Review.
-
Mechanisms and novel therapeutic roles of bitter taste receptors in diseases.Theranostics. 2025 Mar 3;15(9):3961-3978. doi: 10.7150/thno.107406. eCollection 2025. Theranostics. 2025. PMID: 40213652 Free PMC article. Review.
-
Hops bitter β-acids have antibacterial effects against sinonasal Staphylococcus aureus but also induce sinonasal cilia and mitochondrial dysfunction.Int Forum Allergy Rhinol. 2025 Mar;15(3):287-302. doi: 10.1002/alr.23487. Epub 2024 Nov 13. Int Forum Allergy Rhinol. 2025. PMID: 39533961 Free PMC article.
-
Savory Signaling: T1R Umami Receptor Modulates Endoplasmic Reticulum Calcium Store Content and Release Dynamics in Airway Epithelial Cells.Nutrients. 2023 Jan 18;15(3):493. doi: 10.3390/nu15030493. Nutrients. 2023. PMID: 36771200 Free PMC article.
-
Effects of Akt Activator SC79 on Human M0 Macrophage Phagocytosis and Cytokine Production.Cells. 2024 May 24;13(11):902. doi: 10.3390/cells13110902. Cells. 2024. PMID: 38891035 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

