Levetiracetam Mechanisms of Action: From Molecules to Systems
- PMID: 35455472
- PMCID: PMC9030752
- DOI: 10.3390/ph15040475
Levetiracetam Mechanisms of Action: From Molecules to Systems
Abstract
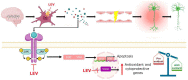
Epilepsy is a chronic disease that affects millions of people worldwide. Antiepileptic drugs (AEDs) are used to control seizures. Even though parts of their mechanisms of action are known, there are still components that need to be studied. Therefore, the search for novel drugs, new molecular targets, and a better understanding of the mechanisms of action of existing drugs is still crucial. Levetiracetam (LEV) is an AED that has been shown to be effective in seizure control and is well-tolerable, with a novel mechanism of action through an interaction with the synaptic vesicle protein 2A (SV2A). Moreover, LEV has other molecular targets that involve calcium homeostasis, the GABAergic system, and AMPA receptors among others, that might be integrated into a single mechanism of action that could explain the antiepileptogenic, anti-inflammatory, neuroprotective, and antioxidant properties of LEV. This puts it as a possible multitarget drug with clinical applications other than for epilepsy. According to the above, the objective of this work was to carry out a comprehensive and integrative review of LEV in relation to its clinical uses, structural properties, therapeutical targets, and different molecular, genetic, and systemic action mechanisms in order to consider LEV as a candidate for drug repurposing.
Keywords: GABAergic system; SV2A; antiepileptic drugs; calcium homeostasis; levetiracetam; neuroinflammation; neuroprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- World Health Organization (WHO) Epilepsy. [(accessed on 6 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy.
-
- Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., Lagae L., Moshé S.L., Peltola J., Roulet Perez E., et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

