Development and Characterization of Eudragit® EPO-Based Solid Dispersion of Rosuvastatin Calcium to Foresee the Impact on Solubility, Dissolution and Antihyperlipidemic Activity
- PMID: 35455489
- PMCID: PMC9025505
- DOI: 10.3390/ph15040492
Development and Characterization of Eudragit® EPO-Based Solid Dispersion of Rosuvastatin Calcium to Foresee the Impact on Solubility, Dissolution and Antihyperlipidemic Activity
Abstract
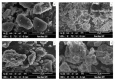
Poor solubility is the major challenge involved in the formulation development of new chemical entities (NCEs), as more than 40% of NCEs are practically insoluble in water. Solid dispersion (SD) is a promising technology for improving dissolution and, thereby, the bioavailability of poorly soluble drugs. This study investigates the influence of a pH-sensitive acrylate polymer, EPO, on the physicochemical properties of rosuvastatin calcium, an antihyperlipidemic drug. In silico docking was conducted with numerous polymers to predict drug polymer miscibility. The screened-out polymer was used to fabricate the binary SD of RoC in variable ratios using the co-grinding and solvent evaporation methods. The prepared formulations were assessed for physiochemical parameters such as saturation solubility, drug content and in vitro drug release. The optimized formulations were further ruled out using solid-state characterization (FTIR, DSC, XRD and SEM) and in vitro cytotoxicity. The results revealed that all SDs profoundly increased solubility as well as drug release. However, the formulation RSE-2, with a remarkable 71.88-fold increase in solubility, presented 92% of drug release in the initial 5 min. The molecular interaction studied using FTIR, XRD, DSC and SEM analysis evidenced the improvement of in vitro dissolution. The enhancement in solubility of RoC may be important for the modulation of the dyslipidemia response. Therefore, pharmacodynamic activity was conducted for optimized formulations. Our findings suggested an ameliorative effect of RSE-2 in dyslipidemia and its associated complications. Moreover, RSE-2 exhibited nonexistence of cytotoxicity against human liver cell lines. Convincingly, this study demonstrates that SD of RoC can be successfully fabricated by EPO, and have all the characteristics that are favourable for superior dissolution and better therapeutic response to the drug.
Keywords: Eudragit® EPO; anti-hyperlipidemic activity; docking; improved solubility; rosuvastatin calcium; solid dispersion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















References
-
- Baghel S., Cathcart H., O’Reilly N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016;105:2527–2544. doi: 10.1016/j.xphs.2015.10.008. - DOI - PubMed
-
- Giri T.K., Kumar K., Alexander A., Ajazuddin , Badwaik H., Tripathi D.K. A novel and alternative approach to controlled release drug delivery system based on solid dispersion technique. Bull. Fac. Pharmacy, Cairo Univ. 2012;50:147–159. doi: 10.1016/j.bfopcu.2012.07.002. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

