Impact of the Genotype and Phenotype of CYP3A and P-gp on the Apixaban and Rivaroxaban Exposure in a Real-World Setting
- PMID: 35455642
- PMCID: PMC9028714
- DOI: 10.3390/jpm12040526
Impact of the Genotype and Phenotype of CYP3A and P-gp on the Apixaban and Rivaroxaban Exposure in a Real-World Setting
Abstract
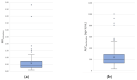
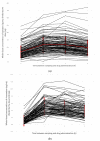
Apixaban and rivaroxaban are the two most prescribed direct factor Xa inhibitors. With the increased use of DOACs in real-world settings, safety and efficacy concerns have emerged, particularly regarding their concomitant use with other drugs. Increasing evidence highlights drug−drug interactions with CYP3A/P-gp modulators leading to adverse events. However, current recommendations for dose adjustment do not consider CYP3A/P-gp genotype and phenotype. We aimed to determine their impact on apixaban and rivaroxaban blood exposure. Three-hundred hospitalized patients were included. CYP3A and P-gp phenotypic activities were assessed by the metabolic ratio of midazolam and AUC0−6h of fexofenadine, respectively. Relevant CYP3A and ABCB1 genetic polymorphisms were also tested. Capillary blood samples collected at four time-points after apixaban or rivaroxaban administration allowed the calculation of pharmacokinetic parameters. According to the developed multivariable linear regression models, P-gp activity (p < 0.001) and creatinine clearance (CrCl) (p = 0.01) significantly affected apixaban AUC0−6h. P-gp activity (p < 0.001) also significantly impacted rivaroxaban AUC0−6h. The phenotypic switch (from normal to poor metabolizer) of P-gp led to an increase of apixaban and rivaroxaban AUC0−6h by 16% and 25%, respectively, equivalent to a decrease of 38 mL/min in CrCl according to the apixaban model. CYP3A phenotype and tested SNPs of CYP3A/P-gp had no significant impact. In conclusion, P-gp phenotypic activity, rather than known CYP3A/P-gp polymorphisms, could be relevant for dose adjustment.
Keywords: DOACs; metabolism; personalized medicine; pharmacogenomics; phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hill N.R., Sandler B., Bergrath E., Milenković D., Ashaye A.O., Farooqui U., Cohen A.T. A Systematic Review of Network Meta-Analyses and Real-World Evidence Comparing Apixaban and Rivaroxaban in Nonvalvular Atrial Fibrillation. Clin. Appl. Thromb. Hemost. 2020;26:1076029619898764. doi: 10.1177/1076029619898764. - DOI - PMC - PubMed
-
- January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Ellinor P.T., Ezekowitz M.D., Field M.E., Furie K.L., et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. - DOI - PubMed
-
- Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

