Predictive Assessment of Quantitative Ultra-Widefield Angiographic Features for Future Need for Anti-VEGF Therapy in Diabetic Eye Disease
- PMID: 35455724
- PMCID: PMC9032777
- DOI: 10.3390/jpm12040608
Predictive Assessment of Quantitative Ultra-Widefield Angiographic Features for Future Need for Anti-VEGF Therapy in Diabetic Eye Disease
Abstract
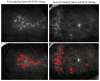
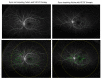
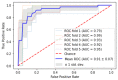
The objective of this study was to identify biomarkers that predict a future need for anti-VEGF therapy in diabetic retinopathy (DR). Eyes with DR that underwent ultra-widefield angiography (UWFA) and had at least a 1 year follow-up were grouped based on future anti-VEGF treatment requirements: (1) not requiring treatment, (2) immediate treatment (within 3 months of UWFA), and (3) delayed treatment (after 3 months of UWFA). Quantitative UWFA features and clinical factors were evaluated. Random forest models were built to differentiate eyes requiring immediate and delayed treatment from the eyes not requiring treatment. A total of 173 eyes were included. The mean follow-up was 22 (range: 11-43) months. The macular leakage index, panretinal leakage index, presence of DME, and visual acuity were significantly different in eyes requiring immediate (n = 38) and delayed (n = 34) treatment compared to eyes not requiring treatment (n = 101). Random forest model differentiating eyes requiring immediate treatment from eyes not requiring treatment demonstrated an AUC of 0.91 ± 0.07. Quantitative angiographic features have potential as important predictive biomarkers of a future need for anti-VEGF therapy in DR and may serve to guide the frequency of a follow-up.
Keywords: anti-VEGF; artificial intelligence; diabetic retinopathy; fluorescein angiography; personalized treatment; predicting anti-VEGF; predictive modeling; quantitative image analysis.
Conflict of interest statement
The authors declare no conflict of interest specifically related to this article. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors have the following financial disclosures: A.C.J.: None; D.D.S.: None; C.M.: None; J.W.: None, S.K.S.: Allergan (R, C); Leica (P), Zeiss (C); Regeneron (R, C); Eyepoint (R, C), Eyevensys (R, C), Novartis (C), Abbvie (C); K.E.T.: Zeiss (R); M.H.: None; J.L.R.: None; J.P.E.: Leica (C, P), Oxurion (C, R), Genentech (C, R), Roche (C, R), Zeiss (C), Alcon (C, R), Novartis (C, R), Aerpio (C, R), Allergan (C, R), Allegro (C), Regeneron (C, R), Alcon (C, R), Boehringer-Ingelheim (C, R), Stealth (C, R), Adverum (C, R).
Figures

References
-
- Mitchell P., Bandello F., Schmidt-Erfurth U., Lang G.E., Massin P., Schlingemann R.O., Sutter F., Simader C., Burian G., Gerstner O., et al. The RESTORE Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. - DOI - PubMed
-
- Wells J.A., Glassman A.R., Ayala A.R., Jampol L.M., Bressler N.M., Bressler S.B., Brucker A.J., Ferris F.L., Hampton G.R., Jhaveri C., et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351–1359. doi: 10.1016/j.ophtha.2016.02.022. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

