Effects of Oxysterols on Immune Cells and Related Diseases
- PMID: 35455931
- PMCID: PMC9031443
- DOI: 10.3390/cells11081251
Effects of Oxysterols on Immune Cells and Related Diseases
Abstract
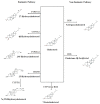
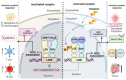
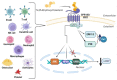
Oxysterols are the products of cholesterol oxidation. They have a wide range of effects on several cells, organs, and systems in the body. Oxysterols also have an influence on the physiology of the immune system, from immune cell maturation and migration to innate and humoral immune responses. In this regard, oxysterols have been involved in several diseases that have an immune component, from autoimmune and neurodegenerative diseases to inflammatory diseases, atherosclerosis, and cancer. Here, we review data on the participation of oxysterols, mainly 25-hydroxycholesterol and 7α,25-dihydroxycholesterol, in the immune system and related diseases. The effects of these oxysterols and main oxysterol receptors, LXR and EBI2, in cells of the immune system (B cells, T cells, macrophages, dendritic cells, oligodendrocytes, and astrocytes), and in immune-related diseases, such as neurodegenerative diseases, intestinal diseases, cancer, respiratory diseases, and atherosclerosis, are discussed.
Keywords: 25-hydroxycholesterol; 7α,25-dihydroxycholesterol; EBI2; LXR; immune cells; immune diseases; oxysterols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

