Live-Cell Imaging of the Contractile Velocity and Transient Intracellular Ca2+ Fluctuations in Human Stem Cell-Derived Cardiomyocytes
- PMID: 35455960
- PMCID: PMC9031802
- DOI: 10.3390/cells11081280
Live-Cell Imaging of the Contractile Velocity and Transient Intracellular Ca2+ Fluctuations in Human Stem Cell-Derived Cardiomyocytes
Abstract
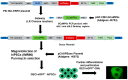
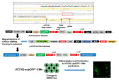
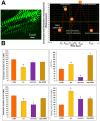
Live-cell imaging techniques are essential for acquiring vital physiological and pathophysiological knowledge to understand and treat heart disease. For live-cell imaging of transient alterations of [Ca2+]i in human cardiomyocytes, we engineered human-induced pluripotent stem cells carrying a genetically-encoded Ca2+-indicator (GECI). To monitor sarcomere shortening and relaxation in cardiomyocytes in real-time, we generated a α-cardiac actinin (ACTN2)-copepod (cop) green fluorescent protein (GFP+)-human-induced pluripotent stem cell line by using the CRISPR-Cas9 and a homology directed recombination approach. The engineered human-induced pluripotent stem cells were differentiated in transgenic GECI-enhanced GFP+-cardiomyocytes and ACTN2-copGFP+-cardiomyocytes, allowing real-time imaging of [Ca2+]i transients and live recordings of the sarcomere shortening velocity of ACTN2-copGFP+-cardiomyocytes. We developed a video analysis software tool to quantify various parameters of sarcoplasmic Ca2+ fluctuations recorded during contraction of cardiomyocytes and to calculate the contraction velocity of cardiomyocytes in the presence and absence of different drugs affecting cardiac function. Our cellular and software tool not only proved the positive and negative inotropic and lusitropic effects of the tested cardioactive drugs but also quantified the expected effects precisely. Our platform will offer a human-relevant in vitro alternative for high-throughput drug screenings, as well as a model to explore the underlying mechanisms of cardiac diseases.
Keywords: CRISPR-Cas9; contractile velocity of cardiomyocytes; drug screening; genetically encoded Ca2+-indicator; hiPSCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

