MicroRNA-like snoRNA-Derived RNAs (sdRNAs) Promote Castration-Resistant Prostate Cancer
- PMID: 35455981
- PMCID: PMC9032336
- DOI: 10.3390/cells11081302
MicroRNA-like snoRNA-Derived RNAs (sdRNAs) Promote Castration-Resistant Prostate Cancer
Abstract
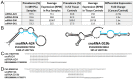
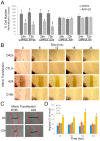
We have identified 38 specifically excised, differentially expressed snoRNA fragments (sdRNAs) in TCGA prostate cancer (PCa) patient samples as compared to normal prostate controls. SnoRNA-derived fragments sdRNA-D19b and -A24 emerged among the most differentially expressed and were selected for further experimentation. We found that the overexpression of either sdRNA significantly increased PC3 (a well-established model of castration-resistant prostate cancer (CRPC)) cell proliferation, and that sdRNA-D19b overexpression also markedly increased the rate of PC3 cell migration. In addition, both sdRNAs provided drug-specific resistances with sdRNA-D19b levels correlating with paclitaxel resistance and sdRNA-24A conferring dasatinib resistance. In silico and in vitro analyses revealed that two established PCa tumor suppressor genes, CD44 and CDK12, represent targets for sdRNA-D19b and sdRNA-A24, respectively. This outlines a biologically coherent mechanism by which sdRNAs downregulate tumor suppressors in AR-PCa to enhance proliferative and metastatic capabilities and to encourage chemotherapeutic resistance. Aggressive proliferation, rampant metastasis, and recalcitrance to chemotherapy are core characteristics of CRPC that synergize to produce a pathology that ranks second in cancer-related deaths for men. This study defines sdRNA-D19b and -A24 as contributors to AR-PCa, potentially providing novel biomarkers and therapeutic targets of use in PCa clinical intervention.
Keywords: CD44; CDK12; cancer; castration-resistant; microRNA; ncRNA; noncoding RNA; prostate; sdRNA; snoRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
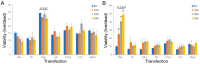

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

